ABSTRACT
Hypertension is a major health issues globally. Multiple genetic and environmental factors are involved in hypertension etiology. Solute carrier family 35 member F3 (SLC35F3) is a type of transporter uptakes thiamin across the cellular and mitochondrial membrane. Recent studies suggested that variations in SLC35F3 are associated with the risk of hypertension; however, studies are limited in Koreans. This study examined the association of the genetic variations in SLC35F3 and the risk of hypertension in Koreans using the Korean Genome Epidemiology Study (Ansan/Ansung study). A total of 8,298 Koreans (males 3,983, females 4,315) were analyzed for their general characteristics, dietary intake, and blood pressure. Twenty-four tagging variations in SLC35F3 were selected and investigated for their association with the risk of hypertension using a sex-stratified approach. Findings suggested that, in males, rs12135117 A allele carriers were at the lower risk for hypertension (adjusted odds ratio, 0.859; 95% confidence interval [CI], 0.740–0.998). In females, rs10910387 TC genotype tended to increase the risk 1.172-fold for hypertension (95% CI, 1.002–1.370). Multiple linear regression models exhibited that rs12135117 A allele was negatively associated with blood pressure in males, and rs10910387 TC genotype had a positive association with blood pressure in females. However, statistical significance for these genetically modified effects was in lacked (Bonferroni's corrected p > 0.002). In conclusion, genetic variation in SLC35F3 is not a decisive prediction marker for hypertension risk in Koreans. Given the rarity of data, more studies are required to evaluate the role of SLC35F3 and thiamin in the hypertension etiology.
-
Keywords: Genetic variation; Hypertension; Koreans; Thiamin transferase
INTRODUCTION
A growing prevalence of hypertension is a global health issue [
1]. Hypertension is one of the major causes of death in Koreans along with cancer, cardiac diseases, pneumonia, and cerebrovascular diseases, and is also an important risk factor for cerebrovascular diseases. According to the 2018 Death Statistics by Statistics Korea, the mortality rate of hypertensive diseases increased by 23.6% in the last decade and has been increasing by 4.9% every year [
2].
Hypertension is grouped into primary and secondary hypertension depending on its cause. Primary hypertension refers to elevated blood pressure that is presented without a clear cause and accounts for approximately 90%–95% of all hypertension case, whereas secondary hypertension refers to the symptoms caused by another condition [
3,
4]. Primary hypertension is reported to result from various and complex combinations of genetic and environmental factors, including food and dietary intake of nutrients [
5].
Thiamin is a water-soluble vitamin, also known as vitamin B1. Thiamin is mainly found in the form of thiamin pyrophosphate (TPP) in the body, which is a coenzyme necessary for energy metabolism and promotes energy production. Hence, thiamin intake and requirements are associated with energy consumption and metabolism [
6,
7]. A reduction in thiamin levels in the body has been reported to be associated with the accumulation of the intermediates in glucose metabolism, including pyruvic acid and lactic acid [
8,
9]. Thiamin has been reported to be involved in cardiovascular diseases etiology, because it promotes dilation of peripheral arteries and increased cardiac output and blood pressure [
10,
11]. A study using induced hypertension rat models proved that supplementation with thiamin reduced the symptoms of hypertension through the regulation of messenger RNA expression associated with the renin-angiotensin system [
12]. It has also been reported that systolic blood pressure (SBP) decreases with body TPP levels [
11]. Additionally, thiamin supplementation improves endothelial cell-dependent vascular dilation and delays the progression of atherosclerosis [
13]. These reports suggest that thiamin is associated with mechanism of blood pressure control.
Dietary thiamin is hydrolyzed into thiamin monophosphate (TMP) and is absorbed in the jejunum and ileum [
14]. TMP is transported across the epithelial membrane into the cytoplasm by the solute carrier family proteins (SLC19A1, SLC19A2, SLC44A4, and SLC22A1) [
14]. Solute carrier family 35 member F3 (SLC35F3) is a protein that mediates the intracellular transport of thiamin. The
SLC35F3 gene is located on chromosome 1q42.2 and includes nine exons (419 kb) encoding a 421-amino acid (46,817 Da) protein [
15]. A Chinese study reported that a rs34032258 C > G single nucleotide polymorphism (SNP) in the
SLC35F3 thiamin transporter gene is associated with the risk of hypertension [
16]. This variant is associated with the levels of diastolic blood pressure (DBP) and blood urea nitrogen and creatinine; subjects with CG and GG genotypes have a higher DBP than those with CC genotype [
16]. A study conducted in the United States reported that genetic variants including rs17514104 and rs16842784 in the
SLC35F3 gene were associated with blood pressure [
10]. These studies suggested the association between the
SLC35F3 and hypertension, yet only limited number of data were reported on such association in a Korean population.
Hypertension is one of the common cardiovascular risk factors as well as critical disease itself. Although efforts are being made to prevent and treat hypertension in Koreans, it is necessary to continuously discover nutritional and genetic factors associated with hypertension. This study investigated the association between hypertension and variation in thiamin transporter
SLC35F3 gene in Koreans. The incidence of hypertension and the subsequent health behaviors differ between males and females [
17,
18]. Therefore, in this study, analyses were conducted in males and females, respectively.
MATERIALS AND METHODS
Cohort description
This study was conducted using the Ansan and Ansung study, a part of the Korean Genome Epidemiology Study (KoGES). The KoGES is a cohort project initiated by the Korea Centers for Disease Control and Prevention to identify the risk factors of chronic diseases including diabetes, hypertension, and cardiovascular diseases that commonly affect Koreans. Data was collected in Ansan (urban) and Ansung (rural area) in 2001 and 2002. More detailed information of KoGES was described in elsewhere [
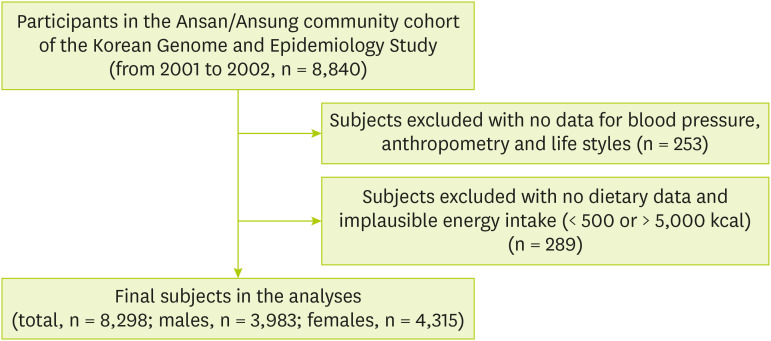
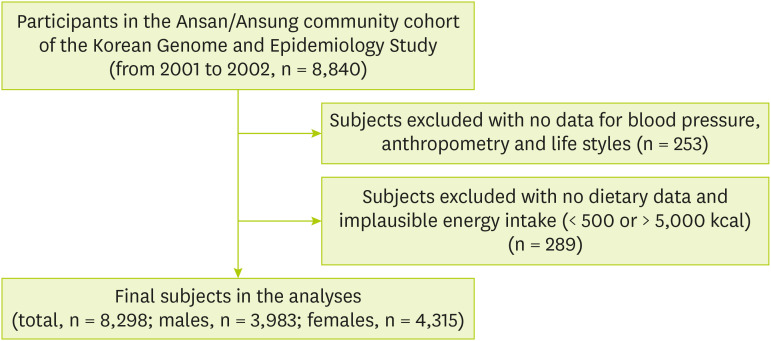
19]. The subject inclusion criteria for this study were as follows. Of the total 8,840 subjects whose genomic and epidemiological information was available, subjects having no data on blood pressure (n = 8), body weight (n = 4), alcohol consumption and smoking status were excluded (n = 144). Subjects having no data on exercise (n = 50), and the education level (n = 47) were also excluded. Additionally, subjects having no data on energy (n = 218) intake and data with unusual energy intake amounts (< 500 kcal or > 5,000 kcal) were excluded (n = 71). Finally, a total of 3,983 males and 4,315 females, i.e., 8,298 subjects were finally selected for analysis (
Figure 1). This study was approved by the Institutional Review Board (40525-201905-BR-017-01) of Keimyung University, Korea.
Figure 1Simplified flow chart of study subject selection.
Data collection
All study data including general, dietary and genotype information were obtained from the KoGES under the permission of the Korea Centers for Disease Control and Prevention. General epidemiological information about the subjects including age, sex, and education level were collected through questionnaires. SBP, DBP, and anthropometric measurements were also collected and analyzed [
19]. The level of physical activity was calculated in metabolic equivalents of task [
20]. Smoking and alcohol drinking status of the subjects was categorized as “Ever (past or current)” and “Never”. The level of education was grouped as follows: “Low” for elementary school graduate or less, “middle” for middle and high school graduate, and “high” for college graduate or more.
Following the 2018 Korean Society of Hypertension Guidelines, subjects were defined a case with hypertension if their SBP was ≥ 140 mmHg or DBP was ≥ 90 mmHg [
21], and/or taking any type of treatment for hypertension [
16].
Data about the energy and nutritional intake of the subjects in the KoGES were collected as follows. Data on nutritional intake were collected using a semi-quantitative food frequency questionnaire (semi-FFQ) [
22]. Frequency of intake (“Almost never,” “Once a month,” “2–3 times a month,” “1–2 times a week,” “3–4 times a week,” “once a day,” “twice a day,” and “3 times a day”) and the average portion (“small,” “medium,” and “large”) of 103 different foods consumed for the last 12 months were recorded by the subjects in the FFQs. From the dietary intake, nutritional consumption was estimated using the database of the Korean Nutrition Society [
22,
23].
Fasting blood samples were collected from the subjects and were used for genotyping. The detailed procedure for genotyping, quality control and imputation were described earlier [
24]. The genotype data was analyzed using 500 ng of genomic DNA and Affymetrix Genome-Wide Human SNP array 5.0 (Affymetrix Inc., Santa Clara, CA, USA). The quality control steps of genotype and imputation were conducted following criteria: If samples with high missing genotype call rate > 4%, high heterozygosity (> 30%) and sex, ethnic mismatch or cryptic relatedness were excluded. Additionally, SNPs with high missing gene call rate (> 5%), minor allele frequency (MAF) of < 0.01, and those not in Hardy-Weinberg equilibrium (HWE, p < 1 × 10
−6) were also excluded. Imputation of genotype data was conducted by IMPUTE software referencing Asian (JPT and CHB) genotype data in HapMap (release 22/NCBI, build 36, and dbSNP build 126) [
25,
26]. After the imputation process, SNPs with genotype information content (info < 0.5), a posterior probability score < 0.90, MAF < 0.01 and HWE p < 1 × 10
−7 were removed from the dataset [
27].
According to the 1000 Genome Project, there are approximately 27,700 genetic variations in the
SLC35F3 gene [
28]. Among them, a total of 336 genetic variations were found and extracted in the genomic data by PLINK (v1.07) [
29]. Using Haploview software (v 4.2), a linkage disequilibrium block (LD block, frequency > 0.1) analysis was performed on 235 of the 336 genetic variations with a MAF ≥ 0.1. A total of 24 tagging loci were found from the analysis [
30]. Therefore 24 taggers were finally selected as genetic markers and used to analyze the correlations between the genetic variations and risk of hypertension. The LD blocks and list of all the markers analyzed in the study are shown in
Supplementary Figure 1 and
Supplementary Table 1.
General characteristics of the subjects were analyzed using the χ2 tests or Student's t-tests taking account of the data type. Logistic regression was used to analyze the correlation between SLC35F3 genotypes and the risk of hypertension. Crude model examined the risk of hypertension caused by a genetic variation without the covariates, and adjusted model examined the risk of hypertension with the covariates including residence area, age, body mass index (BMI, kg/m2), smoking and alcohol use, educational and physical activity level, and total energy, sodium, potassium, and thiamin intake. The association between genotypes and hypertension were addressed in odds ratios and 95% CIs. To provide the more information of the influence of genetic variants on blood pressure, multiple linear regression analyses were performed with the variants showed an association with risk for hypertension additionally. The multicollinearity among the covariates were examined with the condition index (all variables < 55). The Bonferroni method was used to address the multiple comparison problem at the statistical significance level of p < 0.002 (0.05/24 is the number of genetic variations analyzed). SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) were used for all statistical analyses.
RESULTS
Table 1 shows the general characteristics of the hypertension and control groups. In males and females, hypertension cases were 1,451 (36.43%) and 1,405 (32.56%), respectively. Both the male and female hypertension subjects had a higher mean age and BMI (all p < 0.001). Male and female normal subjects were more likely living in the rural area (both p < 0.001). Male cases were more likely to be a drinker (p < 0.001), yet the ratio of smokers did not differ between phenotype. The hypertensive group also tended to have higher level of physical activity than the control group. In both males and females, subjects with lower level of education were more likely have a hypertension (both p < 0.001). The dietary nutrients consumption differed by hypertension phenotype in males and females. The total calorie intake and dietary intake of potassium, sodium and thiamin did not differ between hypertensive and non-hypertensive males. In contrast, females with hypertension had a significantly lower level of intake for total energy (p < 0.001) and potassium (p < 0.001) than those without hypertension. Female hypertension group consumed significantly lower level of thiamin (p < 0.001), although the level of consumption satisfied the recommended nutrient intake (RNI, 1.1 mg/day) [
31]. As these various demographic, lifestyle, and dietary factors may be associated with the risk of hypertension, those were considered as covariates in statistical models analyzing the association between
SLC35F3 variants and the risk of hypertension.
Table 1 General characteristics of the study population taking into account the sex and hypertension phenotypes
Table 1
|
Variables |
Males (n = 3,983, 100%) |
Females (n = 4,315, 100%) |
|
Normal |
Hypertension |
p value |
Normal |
Hypertension |
p value |
|
No. of subjects |
2,352 (63.57) |
1,451 (36.43) |
|
2,910 (67.44) |
1,405 (32.56) |
|
|
Residence area |
|
|
< 0.001 |
|
|
< 0.001 |
|
Ansan (urban) |
922 (36.41) |
714 (49.21) |
1,214 (41.72) |
908 (64.35) |
|
Ansung (rural) |
1,610 (63.59) |
737 (50.79) |
1,696 (58.28) |
503 (35.65) |
|
Age (yr) |
50.29 ± 8.42 |
53.93 ± 8.82 |
< 0.001 |
50.29 ± 8.47 |
57.22 ± 8.22 |
< 0.001 |
|
Body mass index (kg/m2) |
23.88 ± 2.83 |
24.97 ± 2.96 |
< 0.001 |
24.39 ± 3.08 |
25.98 ± 3.33 |
< 0.001 |
|
Physical activity (MET-h) |
23.30 ± 14.45 |
24.93 ± 15.63 |
0.021 |
21.95 ± 13.62 |
24.21 ± 15.68 |
0.045 |
|
Alcohol drinking |
|
|
< 0.001 |
|
|
< 0.001 |
|
Never drinker |
498 (19.67) |
222 (15.30) |
2,013 (69.18) |
1,076 (76.58) |
|
Ever drinker |
2,034 (80.33) |
1,229 (84.70) |
897 (30.82) |
329 (23.42) |
|
Tobacco smoking |
|
|
0.274 |
|
|
0.957 |
|
Never smoker |
477 (18.84) |
294 (20.26) |
2,771 (95.22) |
1,336 (95.09) |
|
Ever smoker |
2,055 (81.16) |
1,157 (79.74) |
139 (4.78) |
69 (4.91) |
|
Education |
|
|
< 0.001 |
|
|
< 0.001 |
|
High |
598 (23.62) |
272 (18.75) |
233 (7.66) |
39 (2.78) |
|
Middle |
1,498 (59.16) |
834 (57.48) |
1,636 (56.22) |
490 (34.88) |
|
Low |
436 (17.22) |
345 (23.78) |
1,051 (36.12) |
876 (62.35) |
|
Nutrient consumption |
|
|
|
|
|
|
|
Total energy (kcal/day) |
2,007.3 ± 578.5 |
2,023.7 ± 597.2 |
0.534 |
1,891.1 ± 621.8 |
1,805.9 ± 631.3 |
< 0.001 |
|
Potassium (mg/day) |
2,529.1 ± 1,058.2 |
2,574.2 ± 1,058.9 |
0.210 |
2,544.5 ± 1,146.5 |
2,394.9 ± 1,149.1 |
< 0.001 |
|
Sodium (mg/day) |
3,339.3 ± 1,627.6 |
3,423.5 ± 1,568.6 |
0.066 |
3,014.7 ± 1,523.7 |
2,937.6 ± 1,546.8 |
0.039 |
|
Thiamin (mg/day) |
1.30 ± 0.52 |
1.33 ± 0.54 |
0.189 |
1.20 ± 0.52 |
1.12 ± 0.52 |
< 0.001 |
Table 2 shows the association between
SLC35F3 variants and the risk of hypertension predicted by logistic regression analyses (refer to
Supplementary Tables 2 and
3 for all analyses results). Among the examined total 24 genetic variants, a genetic variation rs12135117 T > A found to be associated with the risk of hypertension in males. Although crude statistical model did not show the significant association, having of rs12135117 variant allele A decreased the risk for hypertension by approximately 14% (95% CI, 0.740–0.998, p = 0.047) in the adjusted model with various demographic, lifestyle, and dietary characteristics of the subjects. In females, the genetic variation rs10910387 T > C in
SLC35F3 showed an association with risk for hypertension. Adjusted statistical model revealed that having of variant TC genotype elevated the risk for disease 1.17 folds (95% CI, 1.002–1.370, p = 0.061). Those genetic variants which have shown the association with blood pressure (
Table 3) were applied for further regression analyses. In males, the presence of rs12135117 A allele was negatively associated with the SBP (β = −1.730, adjusted r
2 = 0.138, p = 0.003) and DBP (β = −0.785, adjusted r
2 = 0.074, p = 0.041). In females, having of TC rs10910387 genotype was positively associated with SBP (β = 1.287, adjusted r
2 = 0.245, p = 0.032) and DBP (β = 0.962, adjusted r
2 = 0.183, p = 0.01). However, significance of modified risk of the genetic variants on risk for hypertension and blood pressure were moderate after correction by Bonferroni's rule (p > 0.002).
Table 2 The association between selected SLC35F3 genetic variations and the risk of hypertension
Table 2
|
Variation |
Control |
Case |
OR_crude (95% CI)*
|
OR_adjusted (95% CI)†
|
p_adjusted‡
|
|
Males |
|
|
|
|
|
|
rs12135117 |
|
|
|
|
|
|
TT |
1,811 (71.52) |
1,076 (74.16) |
Reference |
Reference |
- |
|
AT |
641 (25.32) |
350 (24.12) |
0.919 (0.791–1.068) |
0.902 (0.773–1.053) |
0.092 |
|
AA |
80 (3.16) |
25 (1.72) |
NA |
NA |
- |
|
AT + AA |
721 (28.48) |
375 (25.84) |
0.875 (0.757–1.013) |
0.859 (0.740–0.998) |
0.047 |
|
Females |
|
|
|
|
|
|
rs7552485 |
|
|
|
|
|
|
TT |
1,093 (41.40) |
495 (38.70) |
Reference |
Reference |
|
|
TC |
1,172 (44.39) |
607 (47.46) |
1.144 (0.990–1.321) |
1.172 (1.002–1.370) |
0.061 |
|
CC |
375 (14.20) |
177 (13.84) |
1.042 (0.847–1.283) |
1.022 (0.816–1.280) |
0.588 |
Table 3 The association between selected SLC35F3 genetic variations and blood pressure
Table 3
|
Variation |
Standardized beta coefficient |
Standard error |
Adjusted r2*
|
95% confidence limit |
p value†
|
|
Males |
|
|
|
|
|
|
|
rs12135117 |
SBP |
−1.730 |
0.576 |
0.138 |
−2.860, −0.600 |
0.003 |
|
A allele |
DBP |
−0.785 |
0.383 |
0.074 |
−1.538, −0.033 |
0.041 |
|
Females |
|
|
|
|
|
|
|
rs10910387 |
SBP |
1.287 |
0.601 |
0.245 |
0.108, 2.466 |
0.032 |
|
TC |
DBP |
0.962 |
0.374 |
0.183 |
0.228, 1.697 |
0.010 |
|
CC |
SBP |
0.625 |
0.860 |
0.245 |
−1.062, 2.313 |
0.468 |
|
DBP |
0.930 |
0.536 |
0.183 |
−0.120, 1.981 |
0.083 |
DISCUSSION
This study examined the whether the variations in the SLC35F3 thiamin transporter gene were associated with the risk of hypertension using the KoGES data—Ansan/Ansung study. Findings suggested that SLF35F3 genetic variations did have the significant modifying effect on the risk of hypertension in Koreans.
Beriberi, Wernicke-Korsakoff syndrome, and subacute cerebellar degenerative diseases are well known for severe disorders of thiamin deficiency. Recently, a growing volume of studies suggested that inadequate thiamin intake also acted as a risk factor for other diseases [
31,
32,
33]. Thiamin deficiency leads to reduced adenosine triphosphate synthesis and transketolase reactions, resulting in reduced synthesis of nicotinamide adenine dinucleotide phosphates and pentose. This deficiency, therefore, impairs fatty and nucleic acid synthesis, neurotransmission, and regulatory functions [
32,
33], and leads to the clinical symptoms including psychological issues, such as loss of appetite, weight loss, numbness, short-term memory loss, and confusion. Cardiovascular symptoms, including hypersensitivity, muscle weakness, and cardiac hypertrophy are also common types of the vitamin deficiency [
32,
33].
Absorbed thiamin in the jejunum and ileum is transported across the cell membrane by
SLC35F3, a thiamin transporter, into the cytoplasm [
7,
15]. It has thus been hypothesized that genetic variations in
SLC35F3 may result in the functional changes of protein, hence modify thiamin levels and diseases risk. A few earlier studies support this idea, as described above: A study with approximately 2,500 Chinese hypertensive patients and normal controls reported that GG genotype carriers of the rs34032258 C > G variant have higher DBP compared to that in CC genotype carriers [
16]. Having the TT genotype of the rs17514104 C > T variant was also associated with an increased risk of hypertension [
10]. It has also been reported that possessing of such altered allele was associated with a risk for decreased vascular tolerance, which has been reported to be a symptom of thiamin deficiency that increases cardiac stroke volume and blood pressure in response to temperature-induced stress [
10].
In this study, the rs12135117 and rs7552485 variants in
SLC35F3 were associated with the risk of hypertension and the level of blood pressure in males and females, although the statistical significance were minimal. This limited association may be resulted from the type of variation investigated. A total of 24 tagging loci were examined in this study, however, they were all genic/upstream transcript and intronic variants. Such type of mutation may contribute to the putative association between variants of
SLC35F3 and the risk of hypertension in this study. Additionally, the level of thiamin intake may contribute to the minimal association between genetic variation and the hypertension in this study population. A nutrient-wide approach study with meta-data from INTERnational Collaborative Study on Macro-/Micronutrients and Blood Pressure and United States National Health and Nutrition Examination Survey reported that dietary thiamin intake was inversely associated with SBP [
34]. A Korean study also suggested that the risk for hypertension was decreased in the individuals with the nutrition adequacy ratio for thiamin ≥ 1.2 [
35]. In current study, males' levels of thiamin consumption were higher than RNI (1.2 mg/day) and there was no difference in the mean thiamin intake between hypertensive and non-hypertensive groups. In females, although hypertension group consumed lower level of thiamin, compared to normal, both phenotypes' thiamin intake satisfied RNI. Such favorable thiamin intake may result in the minimal effect of genetic variation on the risk for hypertension and blood pressure regulation. However, again, the statistical significance of the genetically modified risk for hypertension by
SLC35F3 variation was modest. These hypotheses are required to be examined with further study.
Hypertension is a common type of disorder in a Korean population. More than 60% Koreans above 70 years old have hypertension and it is a risk factor for other cardiovascular diseases [
36]. To the best of our knowledge, this is the first study analyzed the association between variations in thiamin transporter gene,
SLC35F3, and hypertension in Koreans. However, the study may harbor few limitations: First, this study used the Ansung/Ansan study data of KoGES data. The examined genetic data did not fully cover the subjects'
SLC35F3 variations and was found to be intronic variations mostly. Furthermore, this study was a cross-sectional design. Although the statistical models used in the analysis accounted for several covariates, not all factors associated with hypertension could be considered. Thus, the results of this study should be interpreted with caution.
This study examined the association between genetic variations of SLC35F3 in thiamin metabolism and hypertension in Koreans, although the statistical significance for findings was limited. Further research is needed to ascertain the effect of the thiamin and related SLC35F3 variants to explore new approach to prevent and treat the hypertension.
National Biobank of KoreaKBN-2019-050
National Research Foundation of Koreahttps://doi.org/10.13039/501100003725NRF-2018R1A1A1A050191552021R1A2C1008635
NOTES
-
Funding: This study was conducted with bioresources from the National Biobank of Korea, the Centers for Disease Control and Prevention, Republic of Korea (KBN-2019-050). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (No. NRF-2018R1A1A1A05019155, 2021R1A2C1008635).
-
Conflict of Interest: The authors declare that they have no competing interests.
SUPPLEMENTARY MATERIALS
Supplementary Table 1
Description of the examined genetic variations of SLC35F3 and their minor allele frequencies in multiple ethnicities
cnr-10-140-s001.xls
Supplementary Table 2
Distribution of genotypes in the studied SLC35F3 genetic variations and their association with hypertension in males
cnr-10-140-s002.xls
Supplementary Table 3
Distribution of genotypes in studied SLC35F3 genetic variations and the association with hypertension in females
cnr-10-140-s003.xls
Supplementary Figure 1
Pairwise linkage disequilibrium blocks among 235 significant single nucleotide polymorphisms in SLC35F3.
cnr-10-140-s004.ppt
REFERENCES
- 1. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol 2020;16:223-237.
- 2. Korean National Statsistical Office. The statistics cause of death [Internet]. 2018. cited 2020 March 16. Available from http://kostat.go.kr
- 3. Charles L, Triscott J, Dobbs B. Secondary hypertension: discovering the underlying cause. Am Fam Physician 2017;96:453-461.
- 4. Staessen JA, Wang J, Bianchi G, Birkenhäger WH. Essential hypertension. Lancet 2003;361:1629-1641.
- 5. Manosroi W, Williams GH. Genetics of human primary hypertension: focus on hormonal mechanisms. Endocr Rev 2019;40:825-856.
- 6. Eshak ES, Arafa AE. Thiamine deficiency and cardiovascular disorders. Nutr Metab Cardiovasc Dis 2018;28:965-972.
- 7. Ortigoza-Escobar JD, Alfadhel M, Molero-Luis M, Darin N, Spiegel R, de Coo IF, Gerards M, Taylor RW, Artuch R, Nashabat M, Rodríguez-Pombo P, Tabarki B, Pérez-Dueñas B. Thiamine Deficiency Study Group. Thiamine deficiency in childhood with attention to genetic causes: survival and outcome predictors. Ann Neurol 2017;82:317-330.
- 8. Beltramo E, Berrone E, Tarallo S, Porta M. Effects of thiamine and benfotiamine on intracellular glucose metabolism and relevance in the prevention of diabetic complications. Acta Diabetol 2008;45:131-141.
- 9. Zenuk C, Healey J, Donnelly J, Vaillancourt R, Almalki Y, Smith S. Thiamine deficiency in congestive heart failure patients receiving long term furosemide therapy. Can J Clin Pharmacol 2003;10:184-188.
- 10. Zhang K, Huentelman MJ, Rao F, Sun EI, Corneveaux JJ, Schork AJ, Wei Z, Waalen J, Miramontes-Gonzalez JP, Hightower CM, Maihofer AX, Mahata M, Pastinen T, Ehret GB, Schork NJ, Eskin E, Nievergelt CM, Saier MH Jr, O'Connor DT. International Consortium for Blood Pressure Genome-Wide Association Studies. Genetic implication of a novel thiamine transporter in human hypertension. J Am Coll Cardiol 2014;63:1542-1555.
- 11. Wilkinson TJ, Hanger HC, Elmslie J, George PM, Sainsbury R. The response to treatment of subclinical thiamine deficiency in the elderly. Am J Clin Nutr 1997;66:925-928.
- 12. Tanaka T, Sohmiya K, Kono T, Terasaki F, Horie R, Ohkaru Y, Muramatsu M, Takai S, Miyazaki M, Kitaura Y. Thiamine attenuates the hypertension and metabolic abnormalities in CD36-defective SHR: uncoupling of glucose oxidation from cellular entry accompanied with enhanced protein O-GlcNAcylation in CD36 deficiency. Mol Cell Biochem 2007;299:23-35.
- 13. Arora S, Lidor A, Abularrage CJ, Weiswasser JM, Nylen E, Kellicut D, Sidawy AN. Thiamine (vitamin B1) improves endothelium-dependent vasodilatation in the presence of hyperglycemia. Ann Vasc Surg 2006;20:653-658.
- 14. Ortigoza-Escobar JD, Molero-Luis M, Arias A, Martí-Sánchez L, Rodriguez-Pombo P, Artuch R, Pérez-Dueñas B. Treatment of genetic defects of thiamine transport and metabolism. Expert Rev Neurother 2016;16:755-763.
- 15. Nishimura M, Suzuki S, Satoh T, Naito S. Tissue-specific mRNA expression profiles of human solute carrier 35 transporters. Drug Metab Pharmacokinet 2009;24:91-99.
- 16. Zang XL, Han WQ, Yang FP, Ji KD, Wang JG, Gao PJ, He G, Wu SN. Association of a SNP in SLC35F3 gene with the risk of hypertension in a Chinese Han population. Front Genet 2016;7:108.
- 17. Kim HC, Oh SM. Noncommunicable diseases: current status of major modifiable risk factors in Korea. J Prev Med Public Health 2013;46:165-172.
- 18. Choi NH, Ahn HS, Lee S. Comparison of health belief levels and health behavior practices according to lifestyle among adults residing in Seoul. Korean J Community Nutr 2011;16:683-696.
- 19. Kim Y, Han BG; KoGES group. Cohort profile: the Korean genome and epidemiology study (KoGES) consortium. Int J Epidemiol 2017;46:e20.
- 20. Min HS, Kim YJ. Quantification of physical activity using epidemiologic questionnaire data in Korea. Public Health Wkly Rep 2012;33:620-624.
- 21. Lee HY. New definition for hypertension. J Korean Med Assoc 2018;61:485-492.
- 22. Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, Park C, Kim DH. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr 2007;61:1435-1441.
- 23. Ministry of Health and Welfare, Korean Nutrition Society. Dietary reference intakes for Koreans 2000. Seoul: Ministry of Health and Welfare; 2000.
- 24. Cho YS, Go MJ, Kim YJ, Heo JY, Oh JH, Ban HJ, Yoon D, Lee MH, Kim DJ, Park M, Cha SH, Kim JW, Han BG, Min H, Ahn Y, Park MS, Han HR, Jang HY, Cho EY, Lee JE, Cho NH, Shin C, Park T, Park JW, Lee JK, Cardon L, Clarke G, McCarthy MI, Lee JY, Lee JK, Oh B, Kim HL. A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet 2009;41:527-534.
- 25. Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007;447:661-678.
- 26. Marchini J, Howie B, Myers S, McVean G, Donnelly P. A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 2007;39:906-913.
- 27. Hwang JY, Sim X, Wu Y, Liang J, Tabara Y, Hu C, Hara K, Tam CH, Cai Q, Zhao Q, Jee S, Takeuchi F, Go MJ, Ong RT, Ohkubo T, Kim YJ, Zhang R, Yamauchi T, So WY, Long J, Gu D, Lee NR, Kim S, Katsuya T, Oh JH, Liu J, Umemura S, Kim YJ, Jiang F, Maeda S, Chan JC, Lu W, Hixson JE, Adair LS, Jung KJ, Nabika T, Bae JB, Lee MH, Seielstad M, Young TL, Teo YY, Kita Y, Takashima N, Osawa H, Lee SH, Shin MH, Shin DH, Choi BY, Shi J, Gao YT, Xiang YB, Zheng W, Kato N, Yoon M, He J, Shu XO, Ma RC, Kadowaki T, Jia W, Miki T, Qi L, Tai ES, Mohlke KL, Han BG, Cho YS, Kim BJ. Genome-wide association meta-analysis identifies novel variants associated with fasting plasma glucose in East Asians. Diabetes 2015;64:291-298.
- 28. 1000 Genomes Project Consortium. Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini JL, McCarthy S, McVean GA, Abecasis GR. A global reference for human genetic variation. Nature 2015;526:68-74.
- 29. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559-575.
- 30. Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005;21:263-265.
- 31. Ministry of Health and Welfare, Korean Nutrition Society. Dietary reference intakes for Koreans 2015. Seoul: The Korean Nutrition Society; 2015.
- 32. Bettendorff L. Thiamin. In Erdman JW, Macdonald IA, Zeisel SH, eds, ddPresent knowledge in nutrition. Hoboken (NJ): McGraw Hill; 2012, pp 261-279.
- 33. Fattal-Valevski A. Thiamine (vitamin B1). J Evid Based Complementary Altern Med 2011;16:12-20.
- 34. Tzoulaki I, Patel CJ, Okamura T, Chan Q, Brown IJ, Miura K, Ueshima H, Zhao L, Van Horn L, Daviglus ML, Stamler J, Butte AJ, Ioannidis JP, Elliott P. A nutrient-wide association study on blood pressure. Circulation 2012;126:2456-2464.
- 35. Koo S, Kim Y, Kim MK, Yoon JS, Park K. Nutrient intake, lifestyle factors and prevalent hypertension in Korean adults: results from 2007–2008 Korean National Health and Nutrition Examination Survey. Korean J Community Nutr 2012;17:329-340.
- 36. The Korean Society of Hypertension. Hypertension factsheet Korea [Internet]. 2019. cited 2020 March 16. Available from http://www.koreanhypertension.org