ABSTRACT
Recently several studies have attempted to investigate the association between vitamin D and microbiota. However, studies have reported inconsistent results. This narrative review aimed to investigate the potential association between vitamin D and microbiota population in the gut by pooling together the results from observational studies and clinical trials. We considered animal and human studies in this field. Several studies have shown the correlation of vitamin D deficiency with microbiota. Furthermore, interventional studies were emerging that vitamin D change the microbiota composition in which leads to an increase in beneficial bacteria, such as Ruminococcaceae, Akkermansia, Faecalibacterium, and Coprococcus while decreases in Firmicutes. Vitamin D could change the microbiota toward decreasing in Firmicutes and increasing in Bacteroidetes. At genera level, vitamin D may connect to some genera of Lachnospiaceae family (e.g., Blautia, Rosburia, Dorea, and Coprococcus). It seems that adequate level of vitamin D is an important factor in improving the composition of the gut microbiota. More studies are needed to confirm possible underling mechanisms.
-
Keywords: Microbiome; Microbiota; Intestines; Vitamin D
INTRODUCTION
Vitamin D deficiency is described as a public health concern globally, which has health consequences in more than one billion people [
1,
2]. Vitamin D is known for its role in calcium-phosphorus homoeostasis and bone metabolism [
2]. Recent evidences have shown the association between hypovitaminosis D and autoimmune disorders [
3], cancers [
4,
5], cardiovascular disease [
6], diabetes mellitus [
7], and infections [
8,
9]. Furthermore, vitamin D deficiency is highly associated with gastrointestinal diseases, including inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and colon cancer [
10,
11]. The presence of vitamin D receptor (VDR) in almost every tissue highlights the importance of vitamin D in biological functions. VDR is expressed in many cells, including muscle, intestinal epithelium, kidney and also in the immune cells [
12,
13]. As VDR is widely expressed in various immune cells, including B cells, T cells and antigen presenting cells, it may demonstrate the immunomodulatory role of vitamin D in different organs such as gut [
13].
Human gut is a host of numerous numbers of microorganisms (about 10
13–10
14) known as microbiota [
14]. Evidence has supported the roles of microbiota in human's immunity and metabolism [
15]. The microbiota includes bacteria, fungi, archaea, protozoa and viruses that act in the human gut as symbiotic or pathogenic [
15,
16]. More than 1,000 different bacterial species have been determined in human gut. The 4 major phyla composed the gut microbiota are at first
Bacteroidetes and
Firmicutes and then
Actinobacteria, and
Proteobacteria [
17]. The alteration in the diversity of gut microbiota, called dysbiosis, can negatively influence gut health. The change in the composition and diversity of gut microbiota depends on many factors like host genetics, environmental factors, diet, antibiotics, pregnancy, and infection [
18,
19,
20,
21]. Among these factors, dietary elements responsible for up to 57% changes in gut microbiota [
22].
Considering that vitamin D deficiency can cause gastrointestinal disease through its immunomodulatory role, recently, a hypothesis has been suggested on an association between vitamin D and gut microbiota. Recent human and animal investigations have shown that vitamin D could alter microbiota composition through increasing the maintenance of gut homeostasis [
23] and decreasing permeability [
24]. However, it is not clear how vitamin D could shift gut microbial communities to achieve these goals. Therefore, the present study reviewed the association between vitamin D and gut microbiota composition. The characteristics of studies are presented in details in
Tables 1 and
2.
Table 1 Summary of animal studies
Table 1
|
Author |
Population |
Sex |
Intervention |
Duration (day) |
Microbiome identification |
Results |
|
Jahani et al. [25] |
Female and male CD-1 mice |
M/F |
5,000 IU D3/kg diet |
During pregnancy, lactation and 3-mon aged |
qRT-PCR targeting 16S rRNA gene |
VDR expression was 50% higher in the offspring in high vitamin D feeding group. |
|
Lower vitamin D levels was correlated with increased pro-inflammatory genes expression at 3-mon age, low vitamin D diet fed mice had lower Bacteroides/Prevotella ratio count at PND 21 although this difference disappeared at adulthood. |
|
Higher level of LPS concentration were seen in vitamin D deficient diet group at adulthood. |
|
Assa et al. [24] |
C57BL/6 mice |
F |
Vitamin D deficient diet |
5 wk |
qPCR targeting 16S rRNA gene |
Higher relative quantities of Bacteroidetes, Firmicutes, Actinobacteria and Gammaproteobacteria: vitamin D deficient mice. |
|
After 10-day injection of Citrobacter rodentium, relative abundance of Gammaproteobacteria and Actinobacteria in vitamin D deficient group. |
|
Assa et al. [26] |
C57BL/6 mice |
F |
Vitamin D deficient diet |
5 wk |
qPCR targeting 16S rRNA gene |
Higher abundance of Bacteroidetes: vitamin D deficient mice. |
|
Relative increase in Gammaproteobacteria was observed in infected mice with LF82. |
|
Ooi et al. [23] |
Cyp KO & VDR KO C57BL/6 mice |
Sex-matched |
1.25 mg/100 g diet |
NR |
qRT-PCR targeting 16S rRNA gene |
Higher abundance of Bacteroidetes and Proteobacteria and lower bacteria from Firmicutes and Deferribacteres phyla was reported in Cyp KO and VDR KO mice compared with wild-type. |
Table 2 Summary of interventional and observational human studies
Table 2
|
Author |
Country |
Study type |
Population |
Sex |
Number |
Dose (IU/day) |
Duration (day) |
Microbiome identification |
Results |
|
Schäffler et al. [27] |
Germany |
Interventional |
Patients with Crohn's disease |
M/F |
17 |
20,000 IU daily from day 1 to day 3, then every second day |
30 |
PCR targeting 16S rRNA gene |
Grater abundance of Alistipes, Barnesiella, unclassified Porphyromonadaceae, Roseburia, Anaerotruncus, Subdoligranulum and an unclassified Ruminococaceae was seen after vitamin D supplementation. |
|
Garg et al. [28] |
London |
Interventional |
Patients with ulcerative colitis |
M/F |
25 |
40,000 IU D3 weekly |
60 |
PCR targeting 16S rRNA gene |
Enterobacteriaceae increased significantly after vitamin D supplementation. |
|
Kanhere et al. [29] |
USA |
Interventional |
Adults with cystic fibrosis |
M/F |
41 |
50,000 IU D3 weekly |
90 |
PCR targeting 16S rRNA gene |
Lactococcus was increased, while Veillonella and Erysipelotrichaceae were decreased. |
|
Sordillo et al. [30] |
USA |
Interventional |
Infants 3–6 mons whose parents had allergies/asthma |
M/F |
333 |
4,000 or 400 IU/day D3 + prenatal vitamins |
NR |
Sequencing of bacterial 16S rRNA gene |
Greater levels of Lachnospiraceae/U. Clostridales, higher frequency of Lachnobacterium, and lower frequency of Lactococcus. |
|
Gominak et al. [17] |
USA |
Interventional |
Neurology patient |
M/F |
90 |
Individualized dose of vitamin D to guarantee a blood level of 60–80 ng/mL |
Over 1,000 |
NR |
NR |
|
Ciubotaru et al. [31] |
USA |
Interventional |
Prediabetes and hypovitaminosis |
M/F |
115 |
50,000 IU/week D3 |
Over 365 |
PCR targeting 16S rRNA gene |
Lower relative abundance of genera belonging to the Lachnospiraceae (e.g., Ruminococcus, Roseburia, Blautia, and Dorea). |
|
Lower abundance of members of Clostridia class. |
|
Cantarel et al. [3] |
USA |
Interventional |
Women with or without relapsing-remitting multiple sclerosis |
F |
- |
5,000 IU/day D3 |
90 |
PCR targeting 16S rRNA gene |
Greater abundance of Faecalibacterium and Enterobacteriaceae, and lower abundance of Ruminococcus. |
|
MS patients (untreated): higher Akkermansia, Faecalibacterium, and Coprococcus genera. |
|
MS patients (treated by GA): higher Janthinobacterium, lower Eubacterium and Ruminococcus. |
|
Talsness et al. [32] |
The Netherlands |
Observational |
One month old infants |
M/F |
913 |
- |
- |
RT-PCR targeting 16S rRNA gene |
A significant negative linear trend between maternal vitamin D supplementation and plasma 25(OH) D concentration and Bifidobacterium spp. was seen. |
|
There was a positive linear trend between quintile groups and Bacteroides fragilis group counts. |
|
In some breast-fed infants vitamin D supplementation leads to lower abundance of Clostridium difficile. |
|
Luthold et al. [33] |
Brazil |
Observational |
Healthy individual |
M/F |
150 |
- |
- |
PCR targeting 16S rRNA gene |
Higher abundance of Provotella and lower abundance of Haemophilus and Veillonella. |
|
Lower abundance of Coprococcus and Bifidobacterium. |
|
Mandal et al. [34] |
Norway |
Observational |
Pregnant women |
F |
60 |
- |
- |
PCR targeting 16S rRNA gene |
Increased Actinobacteria/Proteobacteria ratio, Actinobacteria/Bacteroidetes ratio, Proteobacteria/Firmicutes ratio, and other Bacteroides. |
|
Thomas et al. [35] |
USA |
Observational |
Older men |
M |
567 |
- |
- |
PCR targeting 16S rRNA gene |
Higher levels of 1,25(OH)2 D were more related to butyrate producing bacteria that are associated with better gut microbial health. |
|
Kassem et al. [36] |
USA |
Observational |
Infants |
M/F |
580 |
- |
- |
PCR targeting 16S rRNA gene |
Prenatal and cord blood vitamin D levels were associated with early life (up to 1 mon) gut microbiota. |
VITAMIN D AND MICROBIOTA: ANIMAL STUDIES
In a study by Assa et al. [
26], a relatively high quantity of Bacteroidetes was found in vitamin D deficient mice. The researchers showed that vitamin D through preserving gut barrier homeostasis and tight-junction building reduces dysbiosis and adherent-invasive
Escherichia coli colonization. Assa et al. [
24] also reported the similar results in another study in which vitamin D deficient mice were more vulnerable to infectious and predisposed to epithelial barrier dysfunction that leads to increasing gut permeability. Jahani et al. [
25] indicated supplementation with vitamin D
3 during pregnancy and lactation had different effects on offspring at different life span. Lower vitamin D levels were related with increased pro-inflammatory genes expression, reduction in VDR at 3-month-aged offspring, lower
Bacteroides/Prevotella ratio at day 21 and higher level of lipopolysaccharides (LPS) concentration in adults. In addition, lower bacteria count was reported in the mice received low vitamin D in comparison to high vitamin D diet. Dysbiosis and increasing of injury in gut following VDR or 1, 25(OH)
2D
3 deficiency has been also reported by Ooi et al. [
23]. They found that vitamin D could regulate the intestinal microbiota while
Bacteroidetes and
Proteobacteria were more abundant in fecal sample of cytochrome P (Cyp) knockout (KO) and VDR KO mice in comparison to wild-type mice. In contrast,
Firmicutes were less abundant in Cyp KO and VDR KO mice. The results of Ooi et al. [
23] is similar to Assa et al. [
24] about the abundance of
Bacteroidetes as Assa et al. [
25] reported the relatively high quantity of
Bacteroidetes,
Firmicutes,
Actinobacteria and
Gammaproteobacteria observed in vitamin D deficient mice in one study and in another one higher relative abundance of
Bacteroidetes.
VITAMIN D AND MICROBIOTA: HUMAN STUDIES
Cantarel et al. [
3] conducted a clinical trial on women with or without relapsing-remitting multiple sclerosis (MS) who were vitamin D insufficient. They reported that after 3 months of vitamin D supplementation (5,000 IU/day), the relative abundance of
Faecalibacterium and
Enterobacteriaceae increased, while in overall the relative abundance of
Ruminococcus decreased. Moreover, after supplementation with vitamin D, untreated MS participants had an increased abundance of
Akkermansia,
Faecalibacterium, and
Coprococcus genera, in comparison with healthy controls and glatiramer acetate-treated MS subjects. Those treated with glatiramer acetate compared to other groups had increases in
Janthinobacterium and decreases in
Eubacterium and
Ruminococcus after vitamin D supplementation [
3]. In a 3-month uncontrolled trial, 1,000 neurological patients received individualized doses of vitamin D to guarantee a blood level of 60–80 ng/mL plus B100 (B complex of 100 mg of all B vitamins except 100 mcg of cyanocobalamin, 100 mcg of biotin, and 400 mcg of folic acid). The authors concluded that these patients did not experience the IBS symptoms during 3 years after stopping the supplementation. Supplementing vitamin D plus all 8 B vitamins led to a change in the intestinal microbiome to normal status in 3 months. This result showed the role of normal intestinal microbiome in reducing pain, sleep disorders, and IBS symptoms through increasing vitamin D and B vitamins level [
17].
In another study, Ciubotaru et al. [
31] conducted a double-blind placebo-controlled randomized trial in men with pre-diabetes and vitamin D deficiency for more than one year. Supplementation with 50,000 IU/week ergocalciferol reduced the relative abundance of several genera of the
Lachnospiraceae (e.g.,
Ruminococcus,
Roseburia,
Blautia, and
Dorea) in high vitamin D quartiles. Another clinical trial also investigated the association between vitamin D supplementation and gut microbiome composition on 333 infants 3 to 6-month-aged. After vitamin D supplementation in pregnant women in 2 different doses of 4,000 IU vitamin D + prenatal vitamins or 400 IU vitamin D + prenatal vitamins, fecal samples from infants were collected and analyzed. In infants with higher cord blood vitamin D levels, the relative abundance of
Lachnospiraceae/
U. Clostridales and
Lachnobacterium was higher, while the relative abundance of
Lactococcus was lower [
30].
According to the study recently published by Garg et al. [
28]
Enterobacteriaceae were significantly increased in patients with ulcerative colitis following 40,000 IU D3/week supplementation for 8 weeks. In another controlled trial on patients with Crohn's disease, 20,000 IU D3 were given for one month. Greater abundance of
Alistipes,
Barnesiella, unclassified
Porphyromonadaceae,
Roseburia,
Anaerotruncus,
Subdoligranulum and an unclassified
Ruminococaceae was reported after vitamin D supplementation [
27]. In a double-blind, randomized, placebo-controlled clinical trial on adults with cystic fibrosis,
Lactococcus was increased, while
Veillonella and
Erysipelotrichaceae were decreased after 12-week supplementation with 50,000 IU D3/week [
29]. In another study 62 fecal sample from healthy infants were collected that thirty-five of them were supplemented with 400 IU of vitamin D per day. Comparative metagenomic analysis was done to investigate the distribution and diversity of infant gut microbiota. The researchers found that vitamin D plays an important role in modifying the infant gut microbiota, especially increase the probiotics types [
37].
Observational studies have also been conducted in this field. In a cross-sectional study designed on 150 healthy individuals, authors demonstrated that higher vitamin D intake was associated with higher abundance of
Provotella and lower abundance of
Haemophilus and
Veillonella. Moreover, the abundance of
Coprococcus and
Bifidobacterium was inversely related to the vitamin D intake [
33]. Another study was conducted to find the correlation between some dietary nutrients and microbiota composition in 60 women, during the second trimester of pregnancy. Results showed that higher vitamin D intake is associated with increased ratio of
Actinobacteria/Proteobacteria,
Actinobacteria/Bacteroidetes,
Proteobacteria/Firmicutes, and other
Bacteroides in pregnant women [
34]. Another cohort study by Talsness et al. [
32] aimed to evaluate the effect of vitamin D supplementation of infant and maternal subject on microbiota composition. A significant negative linear trend between maternal vitamin D supplementation and plasma 25(OH) D concentration and
Bifidobacterium spp. was seen. In some breast-fed infants, vitamin D supplementation leads to lower abundance of
Clostridium difficile. In a cross-sectional study of 567 old men, higher levels of 1,25(OH)
2 D were more related to butyrate producing bacteria that are associated with better gut microbial health [
35]. In a birth cohort study, prenatal and cord blood vitamin D levels were associated with early life (up to 1 month) gut microbiota [
36]. Recently, a review highlighted the therapeutic potential of vitamin D/VDR in the gut microbiota modulation and anti-inflammatory effects in IBD [
38].
CRITICAL APPRAISAL OF EVIDENCE
Reviewing the studies showed that the normal microbiota makes up of 4 main phyla (
Bacteroidetes, Firmicutes, Proteobaceria, and
Actinobacteria) [
17] in which many factors including diet could change their balance [
39]. Gut microbiota plays an important role in health and disease and now is considered as a separate human organ that affect the other organs [
40]. The 2 main bacteria phyla in human feces are
Bacteroidetes and
Firmicutes. Other dominant phyla with less relative abundance are
Proteobaceria, and
Actinobacteria [
17,
41]. To make a better view about the results, we considered microbiota in the phylum to genus level. In this section of review, we discuss the probable effect of vitamin D on gut microbiome in the phylum to genus level.
In phyla level, one study has shown that supplementation with vitamin D may change the microbiota composition with reducing in phylum
Firmicutes [
31]. Two other interventional studies have reported inconsistence results in which
Firmicutes genus increased in one study while, the population of
Firmicutes decreased in the other study [
3,
30]. Although some studies showed increased
Firmicutes genus, it has been reported that this genus is known as butyrate producers and anti-inflammatory [
42]. In an observational study, Luthold et al. [
33], have shown a reduction in
Firmicutes phyla while Mandal et al. [
34], have reported an increase in
Proteobaceria/Firmicutes ratio. However, in both studies, the population of phylum
Bacteroidetes increased. There is a hypothesis in which changing in microbiota composition to higher level of
Bacteroidetes and lower level of
Firmicutes would benefit the host while increasing in
Firmicutes may leads to gut barrier dysfunction [
43,
44].
The other 2 dominant phyla are
Proteobaceria, and
Actinobacteria, which seems to present pro-inflammatory and anti-pathogenic properties. One study has reported increase in
Proteobaceria after vitamin D supplementation [
3] and the other showed increase in
Actinobacteria/Proteobaceria,
Actinobacteria/Bacteroidetes, and
Proteobaceri/Firmicutes ratio with higher dietary intake of vitamin D [
34] while Luthold et al. [
33] showed inverse relationship between some
Proteobaceria and
Actinobacteria genus and serum levels of vitamin D. This controversy may be explained by differences in study design. Luthold et al. [
33], conducted his study in a cross-sectional design which is not strong for identifying causal relationships.
In genus level, all genera that their changes have been reported in the studies were as follows:
Lactococcus,
Blautia,
Rosburia,
Ruminococcus,
Dorea,
Faecalibacterum,
Coprococcus,
Veillonella,
Subdoligranulum,
Erysipelotrichaceae,
Eubacterium,
Anaerotruncus,
C. difficile (phylum
Firmicutes),
Provotella,
Alistipes,
Barnesiella,
Porphyromonadaceae (phylum
Bacteroidetes),
Haemophilus,
Janthinobacterium,
Enterobacteriaceae (phylum
Proteobaceria),
Bifidobacterium (phylum
Actinobacteria), and
Akkermansia (phylum
Verrucomicrobia). Among these genera
Blautia,
Rosburia,
Dorea, and
Coprococcus are all from family
Lachnospiaceae. One study showed significant reduction in abundance of
Blautia,
Rosburia,
Ruminococcus, and
Dorea after vitamin D supplementation [
31] which all are associated with increasing gut permeability and inflammation [
45]. In another study by Cantarel et al. [
3] supplementation with vitamin D
3 in MS women lead to increase in abundance of
Akkermansia,
Faecalibacterum, and
Coprococcus (family
Lachnospiaceae) which
Coprococcus and
Faecalibacterum have known as butyrate producers and may be anti-inflammatory [
42].
Akkermansia, another increased genus is a mucin-degrading bacteria [
46]. Sordillo et al. [
30] concluded that higher vitamin D level is correlated with higher abundance of
Lachnospiaceae/
U.
Clostridales. Multivariate analysis showed increasing
Lachnobacterium and decreasing
Lactococcus abundance. According to this research, low vitamin D level is associated with dysbiosis and inflammation progression. Contrary to the results of this study about
Lactococcus, 2 studies reported an increase in
Lactococcus, which related to positive gut health, after supplementation with vitamin D [
29,
33]. On the other hand, based on Luthold et al. [
33], the abundance of
Bifidobacterium inversely related to the vitamin D intake. In line with this finding, the abundance of
Bifidobacterium spp. was inversely related to maternal plasma 25(OH) D concentration in observational study by Talsness et al. [
32].
Bifidobacterium and lactic acid bacteria like
Lactococcus are both known for their potential probiotic effects. Although the results of this review are partly associated with the prebiotic properties of vitamin D, are not confirmed by the contradictory nature of the studies and there is a need for further studies focusing on probiotic bacteria.
Luthold et al. [
33] showed higher abundance of
Provotella and lower abundance of
Coprococcus,
Haemophilus and
Veillonella in the highest vitamin D intake tertile which is consistent with Kanhere et al.'s study [
47] on the
Veillonella which was known as cause of many infections [
47]. Results about
Coprococcus are inconsistent with
Cantarel study which could be due to its cross-sectional design or the other factors for example the probable effect of MS on intestinal microbiota. Moreover, results of this research showed the lowest tertile of vitamin D intake correlated with increasing in LPS level that likely due to increase in gram negative bacteria (
Haemophilus &
Proteobacteria) which have LPS in their outer membrane. As mentioned above, in the Mandal et al.'s research [
34], vitamin D intake was associated with increasing the
Actinobacteria/Proteobacteria,
Actinobacteria/Bacteroidetes,
Proteobacteria/Firmicutes ratio, and other
Bacteroides in pregnant women. On the other hand, higher vitamin D intake may decreases microbiome diversity. It has been known that reduction in microbiota variety is related to some diseases including IBD [
48], obesity [
20], autism [
49], and allergy [
50]. Besides higher intake of vitamin D changes the microbiome toward increasing
Actinobacteria and
Proteobacteria abundance at phyla levels. These 2 phyla presented anti-pathogenic properties [
34]. To be noted that meat and other animal products are important sources of vitamin D and several publications have reported the effect of meat on microbiota [
51]. These relationships may be explained with antimicrobial characteristics of vitamin D that encompass certain groups of bacteria. Therefore, higher intake of vitamin D might cause an increase in probable pathogens [
34]. The observed contradiction in findings may be the result of inaccurate vitamin D assessment method (food frequency questionnaire) used in this research.
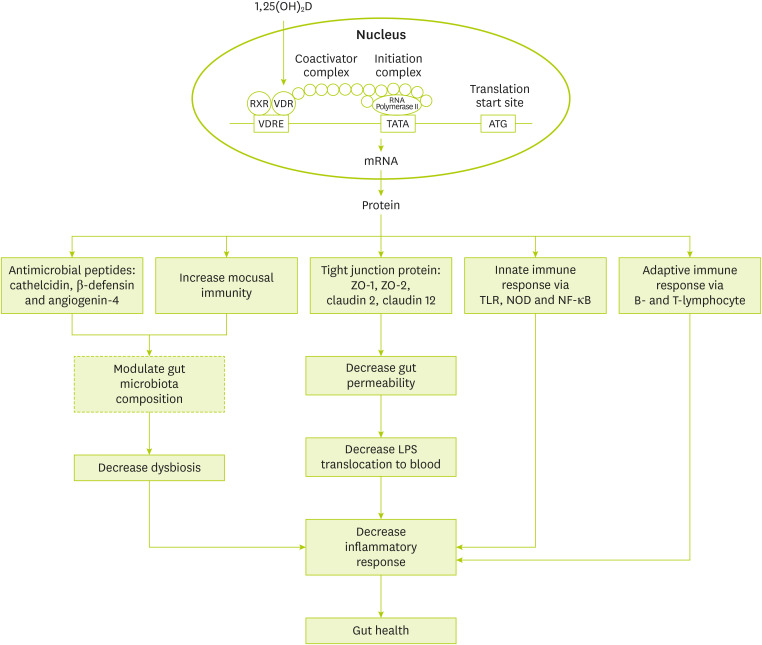
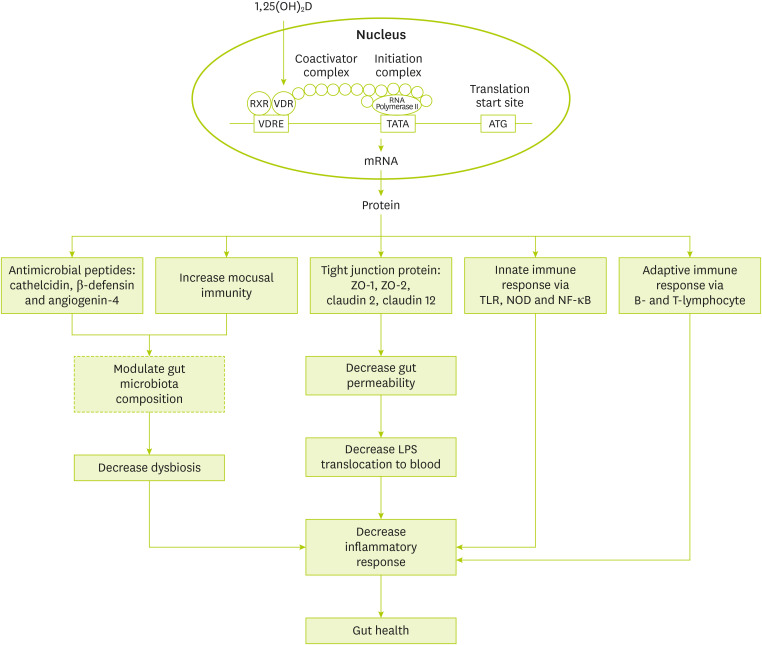
Suggested mechanisms for the role of vitamin D in gut health is shown in
Figure 1. Generally, vitamin D effects are as follows: gene expression modulation of anti-microbial peptides like cathelicidin and β-defensin [
52], gene expression modulation of tight junction proteins like zonulin occluden-1, zonulin occluden-2, claudin 2, and 12 [
53], regulation of innate immune system via gene expression modulation of toll-like receptor 2 and nucleotide-binding oligomerization domain 2 and adaptive immune system via modulation of B- and T-lymphocyte function [
52,
54].
Figure 1
Suggested mechanisms for the association of vitamin D and microbiota.
RXR, retinoid X receptor; VDR, vitamin D receptor; VDRE, vitamin D response element; ZO, zonula occludens; TLR, Toll-like receptor; NOD, nucleotide oligomerization domain; NF-κB, nuclear factor kappa B; LPS, lipopolysaccharides.
CONCLUSION
This study reviewed the data of literatures that investigated the association between vitamin D and gut microbiota. In observational studies, the association of vitamin D deficiency with dysbiosis has been reported. Furthermore, interventional studies were emerging that vitamin D change the microbiota composition in which leads to increase in beneficial bacteria, such as Ruminococcaceae, Akkermansia, Faecalibacterium, Lactococcus, and Coprococcus while decreases in some genera from Firmicutes.
There is scarcity of research on the association between vitamin D and microbiota composition. It seems appropriate dose of vitamin D can alter the gut microbiota with increase in Bacteroidetes and decrease in Firmicutes. At genera level, vitamin D may connect to some genera of Lachnospiaceae family (e.g., Blautia, Rosburia, Dorea, and Coprococcus). Therefore, maintaining the appropriate amount of vitamin D in the body seems to have beneficial effects on the composition of the gut microbiota.
NOTES
-
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
-
Conflict of Interest: The authors declare that they have no competing interests.
REFERENCES
- 1. Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord 2017;18:153-165.
- 2. Bashir M, Prietl B, Tauschmann M, Mautner SI, Kump PK, Treiber G, Wurm P, Gorkiewicz G, Högenauer C, Pieber TR. Effects of high doses of vitamin D3 on mucosa-associated gut microbiome vary between regions of the human gastrointestinal tract. Eur J Nutr 2016;55:1479-1489.
- 3. Cantarel BL, Waubant E, Chehoud C, Kuczynski J, DeSantis TZ, Warrington J, Venkatesan A, Fraser CM, Mowry EM. Gut microbiota in multiple sclerosis: possible influence of immunomodulators. J Investig Med 2015;63:729-734.
- 4. Gaksch M, Jorde R, Grimnes G, Joakimsen R, Schirmer H, Wilsgaard T, Mathiesen EB, Njølstad I, Løchen ML, März W, Kleber ME, Tomaschitz A, Grübler M, Eiriksdottir G, Gudmundsson EF, Harris TB, Cotch MF, Aspelund T, Gudnason V, Rutters F, Beulens JW, van ’t Riet E, Nijpels G, Dekker JM, Grove-Laugesen D, Rejnmark L, Busch MA, Mensink GB, Scheidt-Nave C, Thamm M, Swart KM, Brouwer IA, Lips P, van Schoor NM, Sempos CT, Durazo-Arvizu RA, Škrabáková Z, Dowling KG, Cashman KD, Kiely M, Pilz S. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One 2017;12:e0170791.
- 5. McDonnell SL, Baggerly CA, French CB, Baggerly LL, Garland CF, Gorham ED, Hollis BW, Trump DL, Lappe JM. Breast cancer risk markedly lower with serum 25-hydroxyvitamin D concentrations ≥60 vs <20 ng/ml (150 vs 50 nmol/L): pooled analysis of two randomized trials and a prospective cohort. PLoS One 2018;13:e0199265.
- 6. Zhang R, Li B, Gao X, Tian R, Pan Y, Jiang Y, Gu H, Wang Y, Wang Y, Liu G. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am J Clin Nutr 2017;105:810-819.
- 7. Park SK, Garland CF, Gorham ED, BuDoff L, Barrett-Connor E. Plasma 25-hydroxyvitamin D concentration and risk of type 2 diabetes and pre-diabetes: 12-year cohort study. PLoS One 2018;13:e0193070.
- 8. Laaksi I, Ruohola JP, Tuohimaa P, Auvinen A, Haataja R, Pihlajamäki H, Ylikomi T. An association of serum vitamin D concentrations < 40 nmol/L with acute respiratory tract infection in young Finnish men. Am J Clin Nutr 2007;86:714-717.
- 9. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Griffiths CJ, Janssens W, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S Jr, Stelmach I, Kumar GT, Urashima M, Camargo CA Jr. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583.
- 10. Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol 2012;9:599-608.
- 11. Gagliani N, Hu B, Huber S, Elinav E, Flavell RA. The fire within: microbes inflame tumors. Cell 2014;157:776-783.
- 12. Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D'Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008;117:503-511.
- 13. Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D3 receptor in the immune system. Arch Biochem Biophys 2000;374:334-338.
- 14. Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science 2012;336:1262-1267.
- 15. Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 2013;13:321-335.
- 16. NIH HMP Working Group. Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C, Baker CC, Di Francesco V, Howcroft TK, Karp RW, Lunsford RD, Wellington CR, Belachew T, Wright M, Giblin C, David H, Mills M, Salomon R, Mullins C, Akolkar B, Begg L, Davis C, Grandison L, Humble M, Khalsa J, Little AR, Peavy H, Pontzer C, Portnoy M, Sayre MH, Starke-Reed P, Zakhari S, Read J, Watson B, Guyer M. The NIH human microbiome project. Genome Res 2009;19:2317-2323.
- 17. Gominak SC. Vitamin D deficiency changes the intestinal microbiome reducing B vitamin production in the gut. The resulting lack of pantothenic acid adversely affects the immune system, producing a “pro-inflammatory” state associated with atherosclerosis and autoimmunity. Med Hypotheses 2016;94:103-107.
- 18. Rodríguez JM, Murphy K, Stanton C, Ross RP, Kober OI, Juge N, Avershina E, Rudi K, Narbad A, Jenmalm MC, Marchesi JR, Collado MC. The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis 2015;26:26050.
- 19. Matijašić BB, Obermajer T, Lipoglavšek L, Grabnar I, Avguštin G, Rogelj I. Association of dietary type with fecal microbiota in vegetarians and omnivores in Slovenia. Eur J Nutr 2014;53:1051-1064.
- 20. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature 2009;457:480-484.
- 21. Tangestani H, Emamat H, Ghalandari H, Shab-Bidar S. Whole grains, dietary fibers and the human gut microbiota: a systematic review of existing literature. Recent Pat Food Nutr Agric 2020;11:235-248.
- 22. Del Chierico F, Vernocchi P, Dallapiccola B, Putignani L. Mediterranean diet and health: food effects on gut microbiota and disease control. Int J Mol Sci 2014;15:11678-11699.
- 23. Ooi JH, Li Y, Rogers CJ, Cantorna MT. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr 2013;143:1679-1686.
- 24. Assa A, Vong L, Pinnell LJ, Avitzur N, Johnson-Henry KC, Sherman PM. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J Infect Dis 2014;210:1296-1305.
- 25. Jahani R, Fielding KA, Chen J, Villa CR, Castelli LM, Ward WE, Comelli EM. Low vitamin D status throughout life results in an inflammatory prone status but does not alter bone mineral or strength in healthy 3-month-old CD-1 male mice. Mol Nutr Food Res 2014;58:1491-1501.
- 26. Assa A, Vong L, Pinnell LJ, Rautava J, Avitzur N, Johnson-Henry KC, Sherman PM. Vitamin D deficiency predisposes to adherent-invasive Escherichia coli-induced barrier dysfunction and experimental colonic injury. Inflamm Bowel Dis 2015;21:297-306.
- 27. Schäffler H, Herlemann DP, Klinitzke P, Berlin P, Kreikemeyer B, Jaster R, Lamprecht G. Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn's disease patients, but not in healthy controls. J Dig Dis 2018;19:225-234.
- 28. Garg M, Hendy P, Ding JN, Shaw S, Hold G, Hart A. The effect of vitamin D on intestinal inflammation and faecal microbiota in patients with ulcerative colitis. J Crohns Colitis 2018;12:963-972.
- 29. Kanhere M, He J, Chassaing B, Ziegler TR, Alvarez JA, Ivie EA, Hao L, Hanfelt J, Gewirtz AT, Tangpricha V. Bolus weekly vitamin D3 supplementation impacts gut and airway microbiota in adults with cystic fibrosis: a double-blind, randomized, placebo-controlled clinical trial. J Clin Endocrinol Metab 2018;103:564-574.
- 30. Sordillo JE, Zhou Y, McGeachie MJ, Ziniti J, Lange N, Laranjo N, Savage JR, Carey V, O'Connor G, Sandel M, Strunk R, Bacharier L, Zeiger R, Weiss ST, Weinstock G, Gold DR, Litonjua AA. Factors influencing the infant gut microbiome at age 3-6 months: findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J Allergy Clin Immunol 2017;139:482-491.e14.
- 31. Ciubotaru I, Green SJ, Kukreja S, Barengolts E. Significant differences in fecal microbiota are associated with various stages of glucose tolerance in African American male veterans. Transl Res 2015;166:401-411.
- 32. Talsness CE, Penders J, Jansen EHJM, Damoiseaux J, Thijs C, Mommers M. Influence of vitamin D on key bacterial taxa in infant microbiota in the KOALA Birth Cohort Study. PLoS One 2017;12:e0188011.
- 33. Luthold RV, Fernandes GR, Franco-de-Moraes AC, Folchetti LG, Ferreira SR. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metabolism 2017;69:76-86.
- 34. Mandal S, Godfrey KM, McDonald D, Treuren WV, Bjørnholt JV, Midtvedt T, Moen B, Rudi K, Knight R, Brantsæter AL, Peddada SD, Eggesbø M. Fat and vitamin intakes during pregnancy have stronger relations with a pro-inflammatory maternal microbiota than does carbohydrate intake. Microbiome 2016;4:55.
- 35. Thomas RL, Jiang L, Adams JS, Xu ZZ, Shen J, Janssen S, Ackermann G, Vanderschueren D, Pauwels S, Knight R, Orwoll ES, Kado DM. Vitamin D metabolites and the gut microbiome in older men. Nat Commun 2020;11:5997.
- 36. Kassem Z, Sitarik A, Levin AM, Lynch SV, Havstad S, Fujimura K, Kozyrskyj A, Ownby DR, Johnson CC, Yong GJ, Wegienka G, Cassidy-Bushrow AE. Maternal and cord blood vitamin D level and the infant gut microbiota in a birth cohort study. Matern Health Neonatol Perinatol 2020;6:5.
- 37. Lei WT, Huang KY, Jhong JH, Chen CH, Weng SL. Metagenomic analysis of the gut microbiome composition associated with vitamin D supplementation in Taiwanese infants. Sci Rep 2021;11:2856.
- 38. Battistini C, Ballan R, Herkenhoff ME, Saad SM, Sun J. Vitamin D modulates intestinal microbiota in inflammatory bowel diseases. Int J Mol Sci 2020;22:362.
- 39. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559-563.
- 40. Goulet O. Potential role of the intestinal microbiota in programming health and disease. Nutr Rev 2015;73(Suppl 1):32-40.
- 41. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012;486:207-214.
- 42. Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottière HM, Doré J, Marteau P, Seksik P, Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 2008;105:16731-16736.
- 43. De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 2010;107:14691-14696.
- 44. Schippa S, Conte MP. Dysbiotic events in gut microbiota: impact on human health. Nutrients 2014;6:5786-5805.
- 45. Barengolts E.. Vitamin D and prebiotics may benefit the intestinal microbacteria and improve glucose homeostasis in prediabetes and type 2 diabetes. Endocr Pract 2013;19:497-510.
- 46. Derrien M, Van Baarlen P, Hooiveld G, Norin E, Müller M, de Vos WM. Modulation of mucosal immune response, tolerance, and proliferation in mice colonized by the mucin-degrader Akkermansia muciniphila
. Front Microbiol 2011;2:166.
- 47. Brook I. Veillonella infections in children. J Clin Microbiol 1996;34:1283-1285.
- 48. Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, Nalin R, Jarrin C, Chardon P, Marteau P, Roca J, Dore J. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 2006;55:205-211.
- 49. Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 2013;8:e68322.
- 50. Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Müller G, Stokholm J, Smith B, Krogfelt KA. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol 2011;128:646-652.e1-5.
- 51. Crowe FL, Steur M, Allen NE, Appleby PN, Travis RC, Key TJ. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: results from the EPIC-Oxford study. Public Health Nutr 2011;14:340-346.
- 52. Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, Kiel DP, Streeten EA, Ohlsson C, Koller DL, Peltonen L, Cooper JD, O'Reilly PF, Houston DK, Glazer NL, Vandenput L, Peacock M, Shi J, Rivadeneira F, McCarthy MI, Anneli P, de Boer IH, Mangino M, Kato B, Smyth DJ, Booth SL, Jacques PF, Burke GL, Goodarzi M, Cheung CL, Wolf M, Rice K, Goltzman D, Hidiroglou N, Ladouceur M, Wareham NJ, Hocking LJ, Hart D, Arden NK, Cooper C, Malik S, Fraser WD, Hartikainen AL, Zhai G, Macdonald HM, Forouhi NG, Loos RJ, Reid DM, Hakim A, Dennison E, Liu Y, Power C, Stevens HE, Jaana L, Vasan RS, Soranzo N, Bojunga J, Psaty BM, Lorentzon M, Foroud T, Harris TB, Hofman A, Jansson JO, Cauley JA, Uitterlinden AG, Gibson Q, Järvelin MR, Karasik D, Siscovick DS, Econs MJ, Kritchevsky SB, Florez JC, Todd JA, Dupuis J, Hyppönen E, Spector TD. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet 2010;376:180-188.
- 53. Zhang YG, Wu S, Sun J. Vitamin D, vitamin D receptor and tissue barriers. Tissue Barriers 2013;1:e23118.
- 54. Owen JL, Mohamadzadeh M. Microbial activation of gut dendritic cells and the control of mucosal immunity. J Interferon Cytokine Res 2013;33:619-631.
 , Hossein Khosravi Boroujeni2
, Hossein Khosravi Boroujeni2 , Kurosh Djafarian3
, Kurosh Djafarian3 , Hadi Emamat4
, Hadi Emamat4 , Sakineh Shab-Bidar5
, Sakineh Shab-Bidar5