ABSTRACT
For patients with citrullinemia type 1, nutritional management is essential to prevent the occurrence of complications associated with hyperammonemia. This report describes a patient who had been receiving nutrition intervention for more than 3 years. A newborn diagnosed with hyperammonemia due to citrullinemia visited Ajou University Hospital and was referred to the nutrition team. After receiving acute treatment, the infant was regularly fed with specialized formula. A protein-restricted diet is recommended for maintaining normal development and achieving long-term survival. Through continuous provision of nutritional intervention, the child showed normal growth and development, and the energy-protein supply was maintained appropriately. This case clearly shows the importance of medical nutrition therapy for patients with citrullinemia.
-
Keywords: Nutrition therapy; Citrullinemia; Hyperammonemia
INTRODUCTION
Citrullinemia is a urea cycle disorder (UCD), which is a congenital disease caused by a deficiency of one enzyme. Ammonia, a product of protein metabolism, is converted to urea through the detoxification reaction of the urea cycle and is released from the body through the urine. However, in UCDs, dystrophy develops and nitrogenous waste accumulates in the body in the form of ammonia, thus causing toxic effects; when ammonia reaches the brain through the bloodstream, it causes irreversible brain damage, coma, and eventually death.
The six essential enzymes used in the urea cycle are as follows: N-acetyl glutamate synthetase, ornithine transcarbamylase, carbamoyl phosphate synthetase, argininosuccinic acid synthetase (ASS), argininosuccinic acid lyase, and arginase. Deficiency in one of these enzymes could result in UCDs. Citrullinemia is categorized into types 1 and 2: type 1 is caused by a deficiency of the ASS enzyme, while type 2 is caused by a deficiency of the mitochondrial transport protein citrine [
1].
We present a clinical nutrition management for a 37-month-old boy with citrullinemia type 1.
CITRULLINEMIA TYPE I
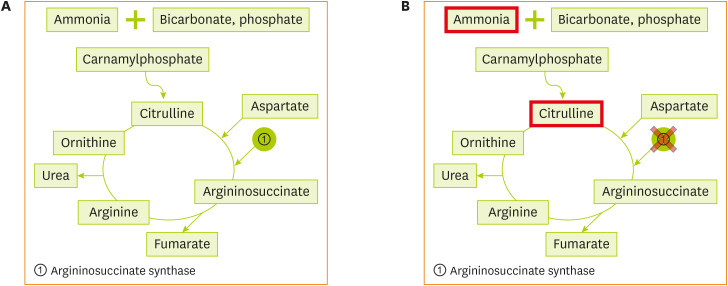
Citrullinemia type 1 [
2] is caused by mutations in the ASS1 gene located on chromosome 9q34.1, which encodes argininosuccinate synthetase, the third enzyme of the urea cycle that catalyzes the formation of argininosuccinic acid from citrulline and aspartic acid. The enzyme is distributed in the tissues, including the liver and fibroblasts. When the level of argininosuccinate synthetase is deficient, as shown in
Figures 1 and
2, citrulline and aspartic acid cannot be converted to argininosuccinic acid, resulting in hyperammonemia and hypercitrullinemia, accompanied by arginine deficiency. This disease is observed in one out of 57,000 newborns worldwide [
3].
Figure 1 (A) Normal urea cycle. (B) Metabolism of citrullinemia.
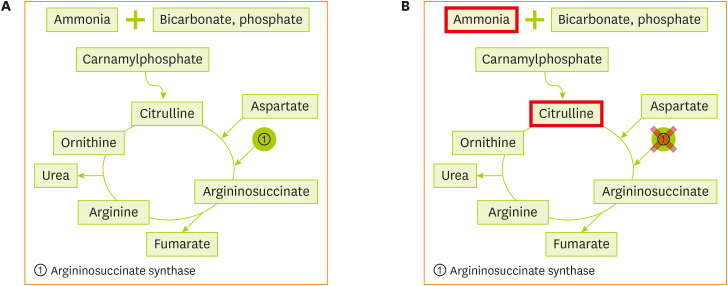
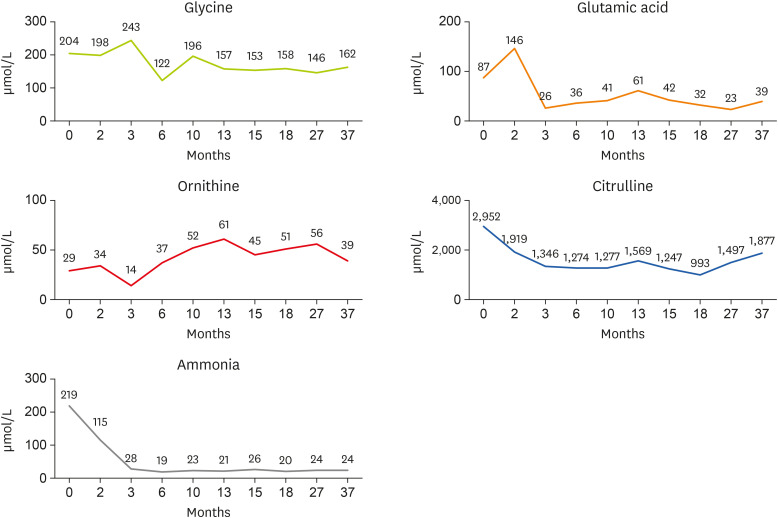
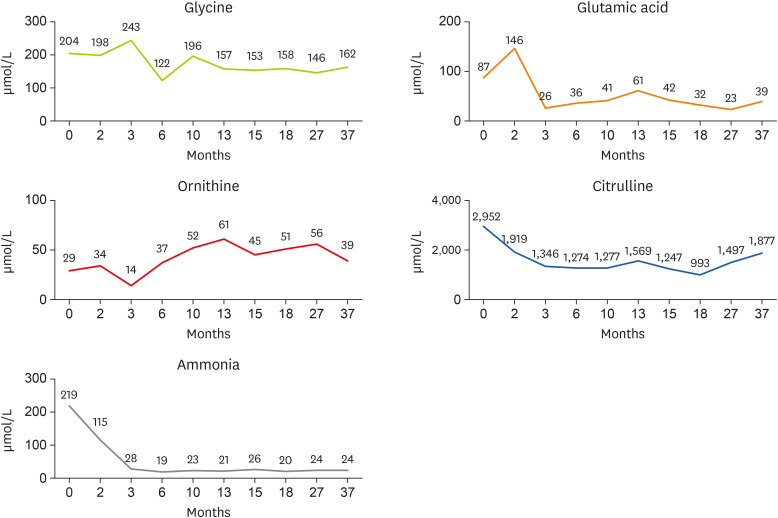
Figure 2 Changes in glycine, glutamic acid, ornithine, citrulline, and ammonia concentrations by age (months).
Clinical symptoms
Infants with citrullinemia type 1, who appear normal but have low residual enzyme concentration, may experience loss of appetite, vomiting, and lethargy due to hyperammonemia within 1 to 2 weeks after birth despite continued lactation, and may progress to coma or apnea and eventually lead to death. It may cause various symptoms depending on the residual enzyme activity, but it is sometimes mistaken for liver disease due to the occurrence of hepatomegaly and increased liver enzyme levels. In delayed type, infants with persistent hyperammonemia experience vomiting, lethargy, headache, tremor, convulsions, and ataxia, accompanied by mental retardation; cerebral atrophy is also observed on brain imaging. Hemiplegia may also be accompanied by cerebral infarction.
Diagnosis
Plasma amino acid analysis shows elevated citrulline levels and hyperammonemia. The excretion of orotic acid is increased, and the blood citrulline concentration is 40 times higher than the normal level. Arginine and ornithine levels are either normal or subnormal, and lysine, glutamine, and alanine levels are increased. Urine organic analysis shows an increase in the citrulline and orotic acid levels. Preimplantation genetic diagnosis, a form of prenatal diagnosis, is possible, and can be performed by analyzing the chorion and amniotic fluid cells.
Treatment
In the acute phase of hyperammonemia, appropriate doses of arginine, sodium benzoate, and phenylbutyrate must be administered. If hyperammonemia is not corrected even after the administration of appropriate drugs, the ammonia level must be normalized by performing a hemodialysis. Additionally, 10% glucose is administered intravenously to ensure that sufficient energy is supplied.
During the recovery period, a low-protein, high-carbohydrate diet (protein intake: 1.0–1.5 g/kg/day) should be maintained, and sodium benzoate or phenylbutyrate can be taken orally to reduce the ammonia levels to 150 μmol/L or less. For long-term treatment, the plasma ammonia level is kept below 100 μmol/L, and arginine, sodium benzoate, and phenylbutyrate are administered to prevent a sudden increase in the ammonia levels; infant formulas containing essential amino acids and a low-protein diet are provided.
Prognosis
The prognosis for intellectual development depends on the age of onset, duration, frequency, and severity of early hyperammonemia [
4,
5].
MEAL PLAN FOR PATIENTS WITH CITRULLINEMIA TYPE 1
Nutrition intervention in acute period
Nutritional management is aimed at providing age-specific energy and protein to meet the infant’s metabolic needs while temporarily minimizing protein intake and preventing endogenous protein catabolism. In
Table 1, for the neonatal period (< 3 months), the recommended energy intake is 125–150 kcal/kg, while the recommended protein intake is 1.25–2.2 g/kg. During this period, a high-carbohydrate and high-fat diet is provided. If the ammonia level is < 100 μmol, protein intake must be resumed to meet the infant’s requirements. Ideally, the period of protein restriction should not exceed 24–48 hours [
6].
Table 1Recommended energy and protein intake for infants and children with citrullinemia according to their growth [1]
Table 1
|
Age |
Nutrient |
|
Protein (g/kg)*
|
Energy (kcal/kg) |
Fluid (mL/kg)†
|
|
Infants |
|
|
|
|
0 to < 3 mon |
1.25–2.20 |
125–150 |
130–160 |
|
3 to < 6 mon |
1.80–2.00 |
120–140 |
130–160 |
|
6 to < 9 mon |
1.60–1.80 |
115–130 |
125–150 |
|
9 to < 12 mon |
1.40–1.60 |
110–120 |
120–130 |
|
Girls and boys |
(g/day) |
(kcal/day) |
(mL/day) |
|
1 to < 4 yr |
16–20 |
945–1,890 |
945–1,890 |
|
4 to < 7 yr |
20–25 |
1,365–2,415 |
1,365–2,415 |
|
7 to < 11 yr |
25–30 |
1,730–3,465 |
1,730–3,465 |
|
Women |
|
|
|
|
11 to < 15 yr |
42–46 |
1,575–3,150 |
1,575–3,150 |
|
15 to < 19 yr |
40–44 |
1,260–3,150 |
1,260–3,150 |
|
≥ 19 yr |
42–46 |
1,785–2,625 |
1,785–2,625 |
|
Men |
|
|
|
|
11 to < 15 yr |
50–55 |
2,100–3,885 |
2,100–3,885 |
|
15 to < 19 yr |
55–60 |
2,200–4,095 |
2,200–4,095 |
|
≥ 19 yr |
60–65 |
2,625–3,465 |
2,625–3,465 |
Arginine (100–600 mL/kg) is administered orally to avoid arginine deficiency and activate the release of ornithine transcarbamylase. Sodium benzoate (250–400 mg/kg/day) or sodium phenylbutyrate (250–500 mL/kg/day) is administered to remove ammonia. Vitamins and trace elements may also be useful.
Nutrition Intervention in long-term period
The goal of long-term nutritional therapy is to prevent hyperammonemia while maintaining normal development. This means limiting protein to a certain amount and providing a high-carbohydrate, high-fat diet. In addition, essential amino acids, vitamins, and minerals must be supplied.
Nutritional recommendations should be individualized according to patients’ growth and weight, and essential amino acids should be supplemented if protein intake is insufficient. Protein intake should be divided into three meals and snacks per day, and prolonged fasting should be avoided. In addition to the three meals per day, planned snacks are necessary. Consumption of pre-meal snacks can reduce the risk of catabolism during the night. Usually, intake of a low-protein diet among patients with citrullinemia type 1 can increase the risk of vitamin and mineral deficiencies. As Fe, Zn, Cu, Ca, and B
12 levels may be deficient, additional supplementation is required [
7]. The levels of essential fatty acids and long-chain polyunsaturated fatty acids can be deficient; therefore, caution is needed. These nutrients can be supplied by feeding the infant with a specialized formula. Currently, the domestic products of specialized formula for children with citrullinemia are UCD-1 and UCD-2. The nutrient compositions of UCD-1 and UCD-2 are listed in
Table 2. They are classified according to age, and the caloric content, calorie density, and micronutrient content differ.
Table 2Composition of specialized formula and standard powdered milk [8] (content per 100 mL)
Table 2
|
Variables |
UCD-1*
|
UCD-2†
|
Standard powdered milk‡
|
|
Age (yr) |
0~3 |
Over 4 |
0–2 |
|
Energy (kcal) |
60 |
81 |
69 |
|
Carbohydrate (g) |
9 |
10 |
7.9 |
|
Sugar (g) |
2 |
10 |
6.1 |
|
Protein (g) |
1 |
3 |
2.1 |
|
Fat (g) |
2.3 |
3.2 |
3.2 |
|
Chlorine (mg) |
39 |
188 |
45 |
|
Choline (mg) |
10 |
19 |
5 |
|
Inositol (mg) |
5.2 |
12 |
4.9 |
|
linolenic acid (g) |
0.5 |
0.7 |
- |
|
Linoleic acid (g) |
- |
- |
0.5 |
|
α-linolenic acid (mg) |
38 |
60 |
53 |
|
L-Carnitine (mg) |
25 |
50 |
1.5 |
|
Taurine (mg) |
7.8 |
17 |
5.6 |
|
L-phenylalanine (mg) |
750 |
1,500 |
- |
|
L-isoleucine (mg) |
1,280 |
2,560 |
- |
|
L-Leucine (mg) |
2,170 |
4,340 |
- |
|
L-lysine (mg) |
1,110 |
2,220 |
- |
|
L-Methionine (mg) |
340 |
680 |
- |
|
L-Threonine (mg) |
750 |
1,500 |
- |
|
L-Tryptophan (mg) |
280 |
560 |
33 |
|
L-Valine (mg) |
1,430 |
2,860 |
- |
|
L-Histidine (mg) |
340 |
680 |
- |
|
L-Aspartic acid (mg) |
- |
- |
- |
|
L-Cystine (mg) |
300 |
600 |
30 |
|
L-Glutamic acid (mg) |
- |
- |
- |
|
Glycine (mg) |
- |
- |
- |
|
L-Proline (mg) |
- |
- |
- |
|
L-Tyrosine (mg) |
880 |
1,760 |
- |
|
L-Serine (mg) |
- |
- |
- |
|
L-Alanine (mg) |
- |
- |
- |
|
L-Arginine (mg) |
- |
- |
55 |
CASE
The patient was born on November 21, 2017 (gestational age: 40+6 weeks, birth weight: 3,100 g, method of delivery: planned Cesarean section) at another hospital. He was brought to the neonatal intensive care unit in December 2017 and was admitted in this unit in January 2018. Regarding the patient's blood test at the time of admission, both the methionine (61.7 μM [cutoff: 60.1 μM]) and citrulline levels increased (2,952.4 μM [cutoff: 67.3 μM]). R/O citrullinemia was diagnosed, and a nutrition management consultation was requested.
Visit 1. Admission (2018-01-17)
Age: 2 months, height: 57 cm, weight: 5.3 kg, weight for length percentile: 103.9%, and Z-score (weight-for-age): 0
At the time of consultation, the level of ammonia in the blood increased but improved at the beginning of hospitalization (
Figure 2). The infant was fed daily with specialized formula, which contained 530 kcal of energy and 10.6 g of protein with a total of 800 mL (100 mL q 3hr), through a feeding bottle.
Nutritional requirements were calculated as 100 kcal/kg of calories and 2 g/kg of protein based on the current body weight. Results of the nutritional intake evaluation demonstrated that the patient met the recommended protein intake, but was unable to achieve the target energy intake. As the ammonia level tends to improve with the use of the UCD formula, the volume of milk per feeding must be increased to 100 kcal/kg according to amount of weight gained, and to continue using the UCD-1 formula until the age of 1 year.
Visit 2. Outpatient (2018-03-07)
Age: 3 months, height: 63.9 cm, weight: 6.8 kg, weight for length percentile: 100.4%, and Z-score (weight for age): 1
The infant was continuously fed with the UCD-1 specialized formula; he received 170 cc of specialized formula every 3 hours, for a total of 1,360 mL, and consumed 810 kcal and 13.6 g of protein per day. The caregiver wanted to increase the volume of infant's milk; however, the current intake was within the maximum allowable range, and the infant gained sufficient weight. Under the guidance of a dietitian, the volume of infant's milk was gradually increased while decreasing the concentration of milk powder or increasing the feeding amount with an extended feeding interval.
Visit 4. Outpatient (2018-06-01)
Age: 6 months, height: 68.5 cm, weight: 7.7 kg, weight for length percentile: 95%, and Z-score (weight for age): −0.5
At the age of 6 months, the patient was fed with rice porridge; since the total daily protein intake obtained from the infant formula might be excessive, a mixed feeding of UCD-1 powder milk and amino acid-free formula (AA-free formula) on a 2:1 ratio was implemented. The nutrient intake analysis showed that the patient’s caloric intake was 89.6 kcal/kg, which was less than the required 100 kcal/kg, and the protein intake was 0.9 g/kg (230 mL each, 4–5 times/day), which was also less than the required intake of 1.5 g/kg (
Table 3). During the process of weaning, proteins with high biological value (meat, etc.) are recommended within the allowed intake range, and the number of formula feedings must be adjusted while increasing the amount of weaning food. In addition, since it was the initial stage of weaning, dietitians provided education about the number of times that the baby should be fed with solid food and powdered milk, and explained the acceptable ingredients and recipes.
Table 3Calorie and protein recommendation and intake until 15 months
Table 3
|
Variables |
2 |
3 |
4 |
6 |
7 |
8 |
10 |
12 |
13 |
15 |
|
Calorie |
|
|
|
|
|
|
|
|
|
|
|
Calorie recommendation (kcal/kg) |
100 |
100 |
120 |
100 |
115 |
115 |
110 |
110 |
110 |
110 |
|
Calorie intake (kcal/kg) |
98.1 |
105.9 |
84.5 |
89.6 |
93.8 |
96.4 |
83.3 |
72.9 |
99.0 |
103.8 |
|
% of calorie intake |
98.1 |
105.9 |
70.4 |
89.6 |
81.5 |
81.6 |
75.7 |
66.3 |
90.0 |
94.3 |
|
Protein |
|
|
|
|
|
|
|
|
|
|
|
Protein recommendation (g/kg) |
2.0 |
2.0 |
2.0 |
1.5 |
1.6 |
1.6 |
1.4 |
1.2 |
1.2 |
1.2 |
|
Protein intake (g/kg) |
1.5 |
2.0 |
1.4 |
0.9 |
1.0 |
1.0 |
1.2 |
0.9 |
1.2 |
1.1 |
|
% of protein intake |
75.0 |
103.0 |
70.0 |
60.0 |
62.5 |
62.5 |
85.7 |
75.0 |
100 |
91.7 |
Visit 7. Outpatient (2018-09-28)
Age: 10 months, height: 73.6 cm, weight: 9.0 kg, weight for length percentile: 97.8%, and Z-score (weight for age): −0.5
As the age increased, the infant started eating baby food and snacks twice a day; accordingly, the ratio of UCD-1 formula to AA-free formula was changed to 1:1. White fish, meat, and tofu were used as weaning foods; the patient was able to properly digest solid foods without complaints of allergic reaction after a meal; the ammonia level remained low; and 75.7% (83.3 kcal/kg) of energy and 85.73% (1.2 g/kg) of protein were consumed compared to nutritional requirements through diet. During the transition to mid-term baby food, the dietitian educated parents to gradually increase the portion size of food, practice chewing, and introduce various foods, and guided them to prepare meals by measuring the allowable amount, protein content, and quantity of each food group.
Visit 9. Outpatient (2019-01-18)
Age: 13 months, height: 79.5 cm, weight: 10.1 kg, weight for length percentile: 97.1%, and Z-score (weight for age): −0.5
As the patient was fed with milk four times a day (600–700 mL/day) and weaning food three times a day (360 g in total), more than 90% of the nutritional requirements were met. As the number of snacks consumed, such as fruits, bread, and biscuits, was increased, a low-protein rice was recommended, and the serving size was calculated under the guidance of a dietitian.
Visit 10. Outpatient (2019-03-15)
Age: 15 months, height: 79.6 cm, weight: 10.6 kg, weight for length percentile 100.9%, and Z-score (weight for age): −0.5
At 15 months of visit, the patient’s diet was changed to soft boiled rice or fried rice, but his condition deteriorated 1 month prior to the outpatient visit due to frequent colds. This also resulted in reduced milk intake; moreover, the patient's weight remained stagnant since the previous visit. The assigned dietitian informed the parents regarding the patient's daily requirement and current intake status, and recommended an increase in the concentration of the specialized formula from 13% to 15% if the intake remained low. As he started going to the daycare center, low-protein rice or side dishes with limited protein content was introduced under the dietitian's guidance to replace regular lunch.
Visit 11. Outpatient (2019-05-24)
Age: 18 months, height: 80.8 cm, weight: 10.5 kg, weight for length percentile: 100%, and Z-score (weight for age): −1
As the child started attending classes in a daycare center, the amount of milk feeding decreased due to environmental adaptation problems or frequent colds, and the patient's weight remained stagnant for more than 3 months. The dietitian explained to the parents how to increase the amount of calories with the use of healthy cooking techniques and taught them on how to calculate the amount of food that could be consumed at daycare centers. The staff of the daycare center were instructed to measure the actual intake of the child, and the dietitian guided all mothers to provide formula milk or snacks at home if the amount of food consumed during lunch at the daycare center was insufficient. Avoiding vegetables (mushrooms and bean sprouts) with high protein content and foods with high biological value protein such as fish, tofu, and meat is recommended. The patient was no longer allowed to consume soup with high protein content, such as Doenjangguk; instead a clear soup was recommended.
Visit 14. Outpatient (2020-02-27)
Age: 27 months, height: 88.7 cm, weight: 13.6 kg, weight for length percentile: 109.2%, and Z-score (weight for age): 0.5
The intake of UCD-1 specialized formula was further increased to 700 mL per day; as the amount of food in each meal was restored, weight gain was observed. As the patient consumed almost all the meals provided for lunch at the daycare center, he ate less protein in the morning and evening food. The preferred foods included soft tofu, eggs, noodles, etc., which contained high quantities of protein; the dietitian guided the parents regarding the appropriate serving size of each food group and the meal intervals. The patient started developing picky about food. As the amount of food intake varied depending on the menu, introduction of various foods that can be eaten with ketchup, was recommended, which was a good strategy to improve the compliance of children. To promote growth and development, including weight gain, intake of 1 g/kg of protein per day was recommended.
Visit 17. Outpatient (2021-01-15)
Age: 37 months, height: 97.6 cm, weight: 15.5 kg, weight for length percentile: 97.6%, and Z-score (weight for age): 0.5
He was a picky eater and refused to eat foods with rough or tough texture, but liked soft-textured foods such as fish, eggs, and tofu. No significant change was observed in the data from the last eating history interview; various recipes and shapes of new foods were introduced to correct unhealthy eating habits. As it was necessary to introduce solid food rather than formula milk considering his age, the dietitian recommended the reduction of the intake of specialized formula or the concentration of powdered milk, and provision of UCD-2 formula at the age of 4 years.
The clinical nutrition management content for patients with citrullinemia cases was summarized as
Table 4.
Table 4Summary
Table 4
|
Visit |
Age (mon) |
Brief comment |
|
- |
Birth |
November 21, 2017 in local hospital (gestational age: 40+6 wk, birth weight: 3,100 g, method of delivery: planned Cesarean section). |
|
Hospitalization and consultation due to the diagnosis of citrullinemia at the birth center, increased laboratory methionine concentration (61.7, cutoff: 60.1 μM) and citrulline concentration (2,952.4, cutoff: 67.3 μM). |
|
1 |
2 |
Ammonia level: 115→49→51; UCD-1 specialized formula feeding started. |
|
2 |
3 |
Continuously fed with the UCD-1 formula. |
|
4 |
6 |
Fed with UCD-1 and AA-free formula on a 2:1 ratio. Fed with rice porridge. |
|
7 |
10 |
Baby food started and the ratio of UCD-1 formula to FFA formula was changed to 1:1. |
|
Practice chewing and introduce various foods. |
|
9 |
13 |
Eating fruits, bread, and biscuits was increased and a low-protein rice was recommended. |
|
Increased consumption of rice gruel (1→2→3 times) with hard texture. |
|
10 |
15 |
Reduced milk or meal intake because of cold. |
|
Started going to the daycare center and eating soft boiled rice or fried rice. |
|
11 |
18 |
Amount of milk feeding decreased and poor weight gain for 5 months. |
|
14 |
27 |
Started to develop picky habits and decreased the amount of snacks and powder milk; slow weight gain observed. |
|
Frequently eating out (rice noodle and Chinese fried pork, etc.) Had protein-controlled breakfast and dinner but had regular lunch at daycare center. |
|
17 |
37 |
Tried eating various food with different texture to correct bad eating habit. |
|
From age 4, considered changing from UCD-1 formula to UCD-2. |
|
Keep good physical development, weight by age > 50%. |
DISCUSSION
Citrullinemia, a congenital metabolic syndrome, is a disease that can lead to fatal results due to the accumulation of toxic substances, including ammonia. However, after the neonatal period, more than 95% of patients survive for a long time. If prolonged elevation of blood ammonia levels occurs, the patient is at higher risk of developing various sequelae such as cerebral palsy, epilepsy, vision loss, and severe intellectual decline. Only 12% of the patients had an IQ of 70 or higher [
9]. A basal ammonia level of < 35 μmol/L is generally recommended in newborns [
10]. For newborns with postnatal hyperammonemia, high-carbohydrate intravenous fluid is administered along with insulin to prevent catabolism [
11,
12]. In patients with severe cases of hyperammonemia, hemodialysis is performed. Moreover, BCAA deficiency can due to treatment with sodium phenylbutyrate, which is used to remove ammonia [
6]. Essential amino acid supplements (Livact) may also be administered to prevent acute and chronic BCAA deficiency.
If the newborns’ swallowing ability is not impaired, a specialized formula is provided for protein control. Essential amino acid supplements can be provided using the UCD-1 and 2 powder milk produced in Korea, and can be used in newborns to adults according to their needs.
A high-carbohydrate, low-protein diet is usually required to prevent catabolism; if this diet is maintained for an extended period of time, children may develop obesity or become overweight. Most of the children with UCD had less muscle mass than the normal group and were overweight for their height [
13]. Only limited studies have reported the growth status of children with citrullinemia, and the recommended daily protein intake to achieve proper growth has not been established.
The citrulline patient who visited the hospital did not have any complications due to early diagnosis and maintained nutritional intake according to the daily nutritional recommendations. As a result, the growth status was good, exceeding the 50th percentile on the growth chart.
If a child with citrullinemia does not develop any complications, then the eating-related functions such as sucking or chewing will not be affected. However, as the patients grow, the intake process is shifted from formula milk, to weaning food, and to general meals; protein intake obtained from typical foods must be controlled. Therefore, they need to be educated on what and how to eat, and how much they should eat in daycare centers and schools, and when eating out.
After weaning, the eating records provided by the daycare centers or obtained based on the eating history of patient's as narrated by their parents were not sufficient to assess the exact amount of calories and protein intake per day. Therefore, it is crucial to educate parents on how to measure and record their diet in the early stages of education.
Citrullinemia is an amino acid metabolism abnormality; the target amount should be adjusted based on the calorie and protein requirements to achieve proper growth and development; continuous monitoring and proper meal planning for essential amino acid supplementation are also required. This case may be helpful in acute and long-term nutritional planning for patients with citrullinemia type 1.
NOTES
-
Conflict of Interest: The authors declare that they have no competing interests.
REFERENCES
- 1. Acosta P, Yannicelli S. The Ross metabolic formula system nutrition support protocols. 4th ed. Columbus (OH): Ross Products Division, Division of Abbott Laboratories; 2001.
- 2. Korea Disease Control and Prevention Agency. Rare disease information citrullinemia. Cheongju: Korea Disease Control and Prevention Agency; 2021.
- 3. Jess G, Thoene M. Rare disease database. Danbury (CT): National Organization for Rare Disorders; 2021.
- 4. Quinonez SC, Thoene JG. Citrullinemia type I. In Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K, eds, ddGeneReviews. Seattle (WA): University of Washington, Seattle; 2016.
- 5. Summar M. Current strategies for the management of neonatal urea cycle disorders. J Pediatr 2001;138(Suppl):S30-S39.
- 6. Häberle J, Boddaert N, Burlina A, Chakrapani A, Dixon M, Huemer M, Karall D, Martinelli D, Crespo PS, Santer R, Servais A, Valayannopoulos V, Lindner M, Rubio V, Dionisi-Vici C. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis 2012;7:32.
- 7. Chong MF. V. Shaw and M. Lawson (editors). Clinical Paediatric Dietetics, Oxford: Blackwell Publishing, 2007. pp. 604 ISBN 9781405134934. Br J Nutr 2008;99:357-389.
- 8. Maeil Co. Ltd. Maeil dairy special disease formula nutrition facts [Internet]. cited 2021 May 20. Available from http://absolute.maeil.com/product/detail.do?cate_seq=4&page=1&searchTag=&nationCode=KR&dpid=A0000024
- 9. Cho JH, Kim CH, Lee KH, Jeon IK, Kim JM, Kang BM. The first successful live birth following preimplantation genetic diagnosis using PCR for type 1 citrullinemia. Obstet Gynecol Sci 2014;57:244-247.
- 10. Summar ML, Mew NA. Inborn errors of metabolism with hyperammonemia: urea cycle defects and related disorders. Pediatr Clin North Am 2018;65:231-246.
- 11. Fryburg DA, Jahn LA, Hill SA, Oliveras DM, Barrett EJ. Insulin and insulin-like growth factor-I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. J Clin Invest 1995;96:1722-1729.
- 12. Wolfe RR. Regulation of skeletal muscle protein metabolism in catabolic states. Curr Opin Clin Nutr Metab Care 2005;8:61-65.
- 13. Singh RH. Nutritional management of patients with urea cycle disorders. J Inherit Metab Dis 2007;30:880-887.