ABSTRACT
Through a survey on dietary intake of children and adolescents with brain lesions, the present study aimed to analyze the current status of nutrient intake and examine the effect of high-protein nutrient drink on their nutritional and muscle statuses. The study participants were 90 juvenile participants aged 8–19 years, with brain lesions. The participants were provided with a protein nutrient drink for 12 weeks and a questionnaire survey on dietary intake was performed to analyze the level of nutrient intake before and after ingestion. The physical measurements were taken to determine the improvements in nutrient and muscle statuses. The results showed that, before the intake of protein nutrient drink as a supplement, the participants exhibited lower height, weight, and body mass index than those of the standard levels of healthy individuals, and the level of nutrient intake through diet was lower than those of the required and recommended levels of nutrient intake for Koreans. Conversely, after the intake of protein nutrient drink for 12 weeks, the level of nutrient intake and physical statuses such as weight showed significant improvements. In addition, the muscle status had undergone approximately 10% of change during the intervention with no significant difference. Thus, to ensure an adequate level of nutrient supply to children and adolescents with brain lesions, there is an urgent need to develop a guideline of nutrient intake. The findings in this study are expected to serve as the basic data for such guidelines.
-
Keywords: Brain; Children with disability; Nutritional status; Proteins; Skeletal muscle
INTRODUCTION
According to the National Survey of Persons with Disabilities conducted by the Ministry of Health and Welfare, the number of persons with disabilities in South Korea was approximately 1,339,496 in 2000 and 2,580,340 in 2017, showing an 51.9% increase in the past decade. Among them, the number of children and adolescents aged below or equal to 17 was 85,000 thus accounting for 3.3% of the total survey population. Although the number of children and adolescents with disabilities is small in view of the total number of persons with disabilities, when considering special requirements regarding their development, the number appears significantly high.
Numerous studies reported that at least 40% of the children with disabilities had low nutrient intake that led to poor growth with a state of malnutrition [
1]. Other studies have reported that 25% had an inappropriate diet and 15%–25% fell below the 5 percentiles of weight for length ratio [
2]. As such, children with disabilities had diverse nutrient problems such as poor growth, anemia, or constipation [
1] attributed to the low levels of use and absorption of nutrients resulting from congenital metabolic disorders, as well as malnutrition and dysfunction related to diet caused by the administration of drugs such as seizure suppressants [
3,
4]. Notably, for most children with disabilities, 30%–50% had a problem related to food intake [
5] with the highest severity displayed by the children with cerebral palsy [
1]. In a study comparing the dietary habits and food intake behaviors between children with disabilities and the control, the food they preferred were different from that preferred by children without disabilities [
6] in shape and size of food [
7]. Notably, the cause of malnutrition in children and adolescents with brain lesions is orofacial dyskinesia due to muscle strain and rigidity caused by a congenital defect that makes food intake difficult [
8]. Juvenile patients with brain lesions causing cerebral palsy have low weight and height compared to children and adolescents with other disabilities [
4,
9,
10,
11]. The most significant cause of these discrepancies is nutrient deficiency and reduced muscle mass [
4,
9,
12].
The recommended level of protein intake in children with disability is approximately 2.0 g/kg per day [
13]. The influencing factors in the estimation of protein requirements in certain age groups or specific participants are the supplied food types and digestibility. The supplied food types determine the amino acid constituents and essential amino acid contents. High contents of essential amino acids are found in animal proteins and soybean proteins that are categorized as high-quality proteins based on high digestibility, while plant proteins show low utility in the body due to the presence of limiting amino acids, as well as similarly low digestibility [
14]. The protein quality is evaluated using a chemical or a biological method. The chemical method is mainly based on the amino acid score (AS), in accordance with the reference proteins of the ideal standard contents of essential amino acids that represent the amino acid requirements estimated for the protein requirements in the human body. The biological method is the chemical method reflecting the rates of digestion and absorption such as the Protein Digestibility Corrected Amino Acid Score (PDCAAS) and the Digestible Indispensable Amino Acid Score (DIAAS) [
15].
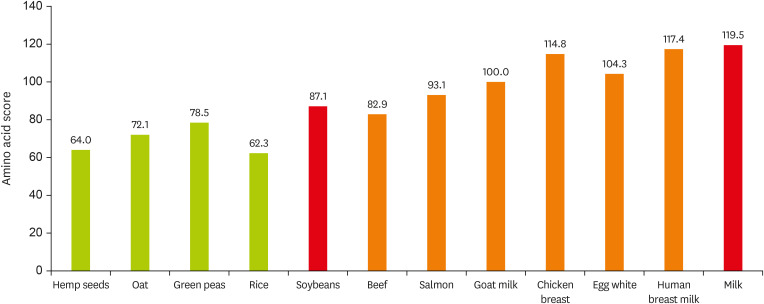
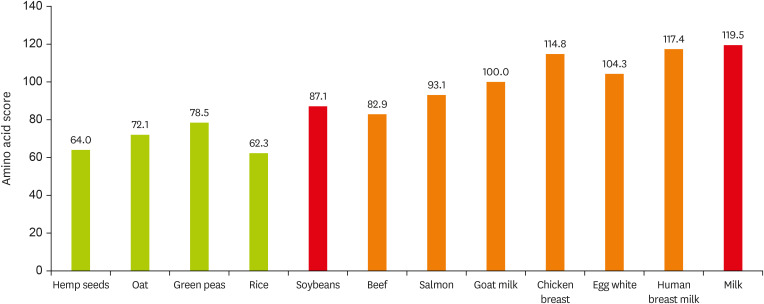
Figure 1 shows the amino acid values for each protein as an example. In South Korea, the intake of animal proteins is below or equal to 50%, a relatively low level compared to that in the western countries, while the intake of plant proteins is high, which predicts a low level of protein utilization efficiency [
16]. Therefore, it is necessary to provide high-quality protein sources to children and adolescents with disabilities showing inadequate protein intake.
Figure 1
Protein amino acid score.
Amino acid score calculated as: mg of Amino Acid in 1 g of Test Protein/mg of Amino Acid in Reference Pattern × 100. Amino acid content data from U.S. Department of Agriculture food data central. Amino acid in reference pattern data from Korean health functional food standards and specifications.
Children and adolescents with disabilities (brain lesions and physical disabilities that pose a difficulty in movement) spend most of their time at home or hospital with minimum outdoor activities, which prevents vitamin D synthesis from sunlight, and the vitamin D required by the body should be obtained via food intake. Moreover, for patients with spastic quadriplegic cerebral palsy, the intake of food produced for enteral nutrition could destroy the balance of nutrients and cause deficiency of micronutrients or vitamins [
17,
18,
19]. Thus, to prevent micronutrient or vitamin deficiency, continuous assessment and monitoring are essential.
The nutrient status in childhood may have a significant impact on the physical and psychological developments in a person’s adult life. The intake of nutrients via diet is essential in physical composition and healthy performance of physical functions [
20].
Thus, overseas countries have conducted various studies on the nutritional characteristics related to chronic diseases, cognitive, and developmental disorders, congenital metabolic disorders or genetic disorders, and the role of each disorder in terms of nutrition to the effect that they have established clinical guidelines [
21]. However, despite the highlighted importance of nutrient management in children and adolescents with disabilities, there is a general lack of studies regarding nutrient management in South Korea and guidelines are yet to be established.
This study investigated the current status of nutrient intake in children and adolescents with brain lesions living at home in the area of Incheon, South Korea. And further identified the effects of the intervention of a 12-week supply of protein nutrient drink on the improvements in health and nutrient statuses as well as in muscle status. The aim was to provide basic data for the development of guidelines regarding nutrient management for children and adolescents with disabilities.
MATERIALS AND METHODS
Study participants and duration of study
The participants in this study were recruited through an advertisement for the members of nongovernmental organization (NGO) group, Happy Link to the juvenile patients with severe disabilities who reside in Incheon, South Korea. As there were concerns regarding reduced comprehension or judgment among the recruited children and adolescents with disabilities, an immediate family or cohabitant (legal representative) was given detailed explanations on the experiment, and signed consent forms were obtained. Among the recruited participants, those unable to perform oral intake, those who are allergic to nuts or dairy products, such as milk, and those on diet control as part of disease management, were excluded. The finally selected participants were children and adolescents with disabilities who were aged 8–19 years, capable of oral intake, and able to drink commercial beverages.
Ninety participants were enrolled in the study, and were provided with a protein nutrient drink for 12 weeks. The nutrient and health statuses before and after the intake of the protein nutrient drink were determined to investigate the level of improvements. This study was conducted with the approval of the Institutional Review Board (IRB; approval number: KHGIRB-20-471).
Demographics
For the demographic data of the participants such as height, weight, date of birth, sex, disability, and treatment, the parents or guardians were requested to complete the questionnaire.
Intake of protein nutrient drink
The amount of protein nutrient drink (Selecs product 125 mL, protein 8 g ; animal protein 6: plant protein 4, leucine 1,000 mg, vitamin D 10 µg, calcium 290 mg; Maeil Health & Nutrition Co., Ltd., Seoul, Korea) provided to the participants was calculated based on the recommended nutrient intake for proteins, calcium, vitamin D, and leucine, according to the Standard Nutrient Intake for Koreans (2020); one pack of drink a day was provided to the participants aged 8–11 years and two packs a day were provided to the participants aged 12–19 years (
Table 1).
Table 1Nutrient of RNI and protein nutrition drinks
Table 1
|
Nutrients |
RNI* (Age 9–11) |
RNI (Age 12–14) |
RNI (Age 15–18) |
Protein drink |
|
Male |
Female |
Male |
Female |
Male |
Female |
125 mL (1 serving) |
250 mL (2 serving) |
|
Protein (g) |
50 |
45 |
60 |
55 |
65 |
55 |
8 |
16 |
|
Vitamin A (µgRE) |
600 |
550 |
750 |
650 |
850 |
650 |
250 |
500 |
|
Vitamin D† (µg) |
5 |
5 |
10 |
10 |
10 |
10 |
10 |
20 |
|
Vitamin B1 (mg) |
0.9 |
0.9 |
1.1 |
1.1 |
1.3 |
1.1 |
0.6 |
1.2 |
|
Vitamin B2 (mg) |
1.1 |
1.0 |
1.5 |
1.1 |
1.4 |
1.1 |
0.45 |
0.9 |
|
Niacin (mgNE) |
11 |
12 |
15 |
15 |
17 |
14 |
3.2 |
6.4 |
|
Vitamin B6 (mg) |
1.1 |
1.1 |
1.5 |
1.4 |
1.5 |
1.4 |
1.0 |
2.0 |
|
Folate (µg) |
300 |
300 |
360 |
360 |
400 |
400 |
85 |
170 |
|
Pantothenic acid (mg) |
4 |
4 |
5 |
5 |
5 |
5 |
1.4 |
2.8 |
|
Calcium (mg) |
800 |
800 |
1,000 |
900 |
900 |
800 |
290 |
580 |
|
Iron (mg) |
11 |
10 |
14 |
16 |
14 |
14 |
5 |
10 |
|
Zinc (mg) |
8 |
10 |
8 |
16 |
10 |
14 |
6 |
12 |
|
Leucine (mg) |
1,900 |
1,800 |
2,700 |
2,400 |
3,200 |
2,400 |
1,000 |
2,000 |
Survey of dietary intake
The 24-hour dietary recall method was used to conduct the survey of dietary intake. The parents or guardians were requested to record the diet in terms of the type and amount of all food and products consumed by the participants in three consecutive days before (week 0) and after (week 12) the intake of protein nutrient drink. The recording log had separate categories for breakfast, morning snack, lunch, afternoon snack, dinner, and others, in which the details of the food name and amount of intake could be recorded as thoroughly as possible to allow the analysis of the current dietary intake of the participants. The recording was performed before and after the intake of protein nutrient drink, and the CAN-Pro (ver. 5.0, 2015; The Korean Nutrition Society, Seoul, Korea) was used to analyze the changes in current nutrient intake as the mean of the recorded intake of three consecutive days.
For the assessment of intake of each nutrient, the measured intake was compared with the recommended nutrient intake (RNI) suggested in the Standard Nutrient Intake for Koreans (2020). The level of energy was tested based on the estimated energy requirement (EER) and the levels of vitamin D and pantothenic acid were tested based on the adequate intake (AI) [
22].
The height and weight were measured, based on which the body mass index (BMI) was calculated to examine the level of growth of the participants before and after the intake of protein nutrient drink. As it was difficult for children with cerebral palsy to stand independently preventing an accurate height measurement, the children were guided to lie down on a flat surface and the height was measured using a tape. The weight was measured in the unit of kg, and when the children had difficulty standing on the scale independently, the parents or guardians were requested to hold the children and stand on the scale, after which the weight of the parents or guardians was subtracted from the measured weight to obtain the weight of the children. The physical measurements of the participants were then compared with the standard height, weight, and BMI of children in the same age group presented in the growth chart created in 2017 by the Korea Disease Control and Prevention Agency. For adolescents aged ≥ 12 years, significant differences in physical measurements were observed between those aged 12–14 years and those aged 15–19 years therefore making a comparison between 3 age groups: 8–11, 12–14, and 15–19 years. In addition, to examine the muscle status, the skeletal muscle mass, fat free mass and body fat mass were measured via the bioelectric impedance analysis (InBody S10; InBody Co., Seoul, Korea). The InBody device, likewise, allowed measurements while the participants were lying down on a surface. For the skeletal muscle mass, while the muscles in the human body are broadly categorized into the cardiac, visceral, and skeletal muscles, the InBody device defined the skeletal muscle mass as the number of voluntary muscles that enable body movements. The fat free mass was the muscle mass excluding the body fat mass, as measured by weight; that is, the amount of minerals, water, and bones, excluding fat. The body fat mass was the total amount of lipids that could be extracted from the adipose and other tissues [
23].
All data collected in this study were analyzed using the SPSS® Package Program version 22 (IBM Corp., Armonk, NY, USA). The results obtained in the survey of dietary intake are presented as mean and standard deviation. To test the significance in the comparison between specific values and standard values of physical traits of participants, the independent t-test was performed. To compare the times before and after the intake of protein nutrient drinks, the paired t-test was performed. The level of significance was set to p < 0.05.
RESULTS
General characteristics of participants and physical measurements
For sex, there were 55 males (61.1%) and 35 females (38.9%). The age distribution was 8–11 years (n = 15, 16.7%), 12–14 years (n = 32, 35.6%) and 15–19 years (n = 43, 47.8%). For the type of disability, brain lesion was the most frequent type (n = 66, 73.3%), followed by brain lesion with other disorders (cognitive, auditory, or visual impairment; n = 18, 20.0%), cognitive disorder (n = 4, 4.4%), and physical and cognitive disorders (n = 2, 2.2%) (
Table 2).
Table 2General characteristics of the participants
Table 2
|
Variable |
Total (n = 90) |
8–11 yr (n = 15) |
12–14 yr (n = 32) |
15–19 yr (n = 43) |
|
Sex |
|
|
|
|
|
Male (%) |
55 (61.1) |
11 (73.3) |
19 (59.4) |
25 (56.8) |
|
Female (%) |
35 (38.9) |
4 (26.7) |
13 (40.6) |
18 (43.2) |
|
Type of disability |
|
|
|
|
|
Cerebral lesion disorder grade 1–3 |
66 (73.3) |
11 (73.3) |
23 (71.9) |
32 (78.4) |
|
Multiple disorder (Brain lesions + Other disorders) |
18 (20.0) |
4 (26.7) |
7 (21.9) |
7 (10.8) |
|
Physical disability |
4 (4.4) |
|
1 (3.1) |
3 (8.1) |
|
Physical disability + Intellectual disability |
2 (2.2) |
|
1 (3.1) |
1 (2.7) |
Furthermore, the physical measurements of the participants before the intake of protein nutrient drink (
Table 3) were compared with the standard height, weight, and BMI of children in the same age group presented in the growth chart created in 2017 by the Korea Disease Control and Prevention Agency. Compared to the standard physical measurements of children in the same age group, the male children with brain lesions aged 8–11 years showed significantly lower weight (p < 0.01), height, and BMI (p < 0.05). The female children had significantly lower height than the standard height (p < 0.05), but the weight and BMI did not show significant differences from the standard values. The male children aged 12–14 years showed significantly lower weight and height than the standard values (p < 0.001) with significantly lower BMI values (p < 0.01). The female children, likewise, showed significantly lower values than the standard values (p < 0.01). The male and female children aged ≥ 15 years showed significantly lower weight and height than the standard values (p < 0.001) with a significantly lower BMI (p < 0.01).
Table 3Anthropometric measurement of disabled children and non-disabled children
Table 3
|
Variable |
8–11 yr (n = 15) |
12–14 yr (n = 32) |
15–19 yr (n = 43) |
|
Non-disabled†
|
Disabled |
Non-disabled |
Disabled |
Non-disabled |
Disabled |
|
Weight (kg) |
|
|
|
|
|
|
|
Male |
35.66 ± 5.19 |
24.93 ± 10.65**
|
53.07 ± 4.40 |
35.39 ± 12.06***
|
64.71 ± 2.31 |
45.51 ± 16.84***
|
|
Female |
34.51 ± 5.09 |
30.50 ± 6.81 |
48.73 ± 2.62 |
36.00 ± 10.09**
|
53.79 ± 0.41 |
35.25 ± 13.79***
|
|
Height (cm) |
|
|
|
|
|
|
|
Male |
138.88 ± 6.71 |
127.73 ± 14.37*
|
161.16 ± 5.43 |
142.32 ± 12.44***
|
172.36 ± 1.46 |
155.20 ± 13.71***
|
|
Female |
138.93 ± 7.48 |
128.75 ± 11.21*
|
156.61 ± 2.34 |
148.26 ± 8.94**
|
160.29 ± 0.42 |
140.69 ± 13.77***
|
|
BMI (kg/m2) |
|
|
|
|
|
|
|
Male |
18.35 ± 0.86 |
14.64 ± 3.38*
|
20.50 ± 0.40 |
17.15 ± 3.93**
|
21.92 ± 0.43 |
18.26 ± 4.93**
|
|
Female |
17.80 ± 0.75 |
18.27 ± 2.14 |
19.98 ± 0.49 |
16.18 ± 3.75**
|
21.01 ± 0.08 |
17.17 ± 4.95**
|
Current nutrient intake of children with brain lesions assessed through dietary survey
From the data of a dietary survey of the 90 children in this study, the cases with < 80% compliance for the intake of protein nutrient drink were viewed as non-compliance and only the cases of ≥ 80% compliance (n = 46) were analyzed for the level of nutrient intake. The daily mean nutrient intake per age group compared to the recommended nutrient intake (%RNI), the estimated energy requirement (%EER), and the adequate intake (%AI) are presented in
Table 4. For the analyzed participants (n = 46), the comparison per nutrient in all age groups showed that, except for vitamin B
1, the nutrient intake was below 100% of the recommended levels. Notably, the calorie intake was very low, < 60% of the EER. The protein intake was < 80% of the RNI. The intake of vitamin D was also < 50% of the RNI.
Table 4%RNI* of nutrient intake for disabled children
Table 4
|
Variable |
Intervention (n = 46) |
8–11 yr (n = 9) |
12–19 yr (n = 37) |
|
0 weeks |
12 weeks |
0 weeks |
12 weeks |
0 weeks |
12 weeks |
|
Energy† (%) |
53.22 ± 16.90 |
53.41 ± 14.22 |
57.40 ± 23.45 |
53.72 ± 20.02 |
52.21 ± 15.15 |
53.34 ± 12.79 |
|
Protein (%) |
76.31 ± 28.65 |
72.87 ± 24.93 |
82.66 ± 45.88 |
76.30 ± 31.49 |
74.77 ± 23.37 |
72.04 ± 23.51 |
|
Vitamin A (%) |
38.76 ± 21.84 |
40.91 ± 24.73 |
38.88 ± 24.79 |
40.90 ± 36.29 |
38.73 ± 21.44 |
40.91 ± 21.71 |
|
Vitamin B1 (%) |
102.89 ± 43.00 |
98.28 ± 39.12 |
107.63 ± 53.57 |
95.92 ± 33.43 |
101.74 ± 40.82 |
98.86 ± 40.78 |
|
Vitamin B2 (%) |
83.49 ± 43.40 |
76.72 ± 33.67 |
98.73 ± 72.12 |
85.90 ± 42.82 |
79.79 ± 33.56 |
74.49 ± 31.36 |
|
Niacin (%) |
50.78 ± 18.36 |
49.44 ± 19.73 |
48.75 ± 18.25 |
55.97 ± 30.49 |
51.27 ± 18.61 |
47.85 ± 16.33 |
|
Vitamin B6 (%) |
78.91 ± 33.06 |
74.44 ± 34.82 |
81.05 ± 24.51 |
85.67 ± 27.82 |
48.39 ± 35.09 |
71.71 ± 36.12 |
|
Pantothenic acid‡ (%) |
73.55 ± 34.22 |
70.42 ± 21.30 |
91.14 ± 59.58 |
71.11 ± 33.64 |
69.27 ± 24.05 |
70.26 ± 17.77 |
|
Folate (%) |
80.95 ± 32.02 |
78.17 ± 22.24 |
82.72 ± 41.98 |
74.94 ± 25.69 |
80.52 ± 29.82 |
78.95 ± 21.64 |
|
Vitamin D‡ (%) |
39.31 ± 52.95 |
26.86 ± 24.63 |
82.56 ± 106.62 |
40.59 ± 40.20 |
28.79 ± 19.92 |
23.52 ± 18.46 |
|
Calcium (%) |
47.57 ± 33.59 |
37.64 ± 17.23 |
61.71 ± 66.51 |
49.98 ± 26.24 |
44.13 ± 19.10 |
37.12 ± 13.59 |
|
Magnesium (%) |
22.17 ± 11.95 |
22.93 ± 12.40 |
28.26 ± 18.84 |
31.30 ± 20.27 |
20.68 ± 9.39 |
20.90 ± 8.90 |
|
Iron (%) |
70.99 ± 32.64 |
74.70 ± 27.98 |
74.05 ± 36.30 |
72.39 ± 30.67 |
70.25 ± 32.18 |
75.26 ± 27.71 |
|
Zinc (%) |
64.16 ± 29.18 |
71.98 ± 23.01 |
74.02 ± 32.05 |
74.41 ± 23.97 |
61.76 ± 28.39 |
71.39 ± 23.08 |
|
Leucine (%) |
96.59 ± 57.47 |
84.52 ± 38.39 |
119.18 ± 96.73 |
104.33 ± 67.49 |
91.09 ± 43.47 |
79.70 ± 26.60 |
For each age group, the participants aged 8–11 years showed < 75% of the RNI for vitamin A, niacin, calcium, magnesium, iron, and zinc, and those aged 12–19 years showed < 75% of the RNI for proteins, vitamin A, niacin, vitamin B6, calcium, magnesium, and zinc.
Changes in the nutrient intake, physical measurements, and body composition of children with brain lesions after the 12-week intake of protein nutrient drink
Changes in nutrient intake
The changes in nutrient intake before and after the 12-week intake of protein nutrient drink are presented in
Table 5. Compared to the %RNI, %EER, and %AI for Koreans, all age groups showed a significant increase in the intake of all nutrients except magnesium (before intake: 22.17% ± 11.95%, after intake: 23.48% ± 12.03%). The result values were also ≥ 100% of the %RNI (except niacin and magnesium) and %EER (except pantothenic acid and vitamin D).
Table 5Change in %RNI† before and after intake nutrition beverage
Table 5
|
Variable |
Intervention (n = 46) |
8–11 yr (n = 9) |
12–19 yr (n = 37) |
|
Before |
After |
Before |
After |
Before |
After |
|
Energy‡ (%) |
53.22 ± 16.90 |
64.28 ± 14.39**
|
57.40 ± 23.45 |
60.64 ± 20.08 |
52.21 ± 15.15 |
65.16 ± 12.85***
|
|
Protein (%) |
76.31 ± 28.65 |
100.31 ± 22.78***
|
82.66 ± 45.88 |
94.54 ± 32.20 |
74.77 ± 23.37 |
101.71 ± 20.20***
|
|
Vitamin A (%) |
38.76 ± 21.84 |
107.00 ± 26.96***
|
38.88 ± 24.79 |
83.98 ± 36.09*
|
38.73 ± 21.44 |
112.61 ± 21.31***
|
|
Vitamin B1 (%) |
102.89 ± 43.00 |
197.05 ± 40.15***
|
107.63 ± 53.57 |
162.58 ± 33.43 |
101.74 ± 40.82 |
205.43 ± 37.39***
|
|
Vitamin B2 (%) |
83.49 ± 43.40 |
144.09 ± 33.38***
|
98.73 ± 72.12 |
129.09 ± 41.77 |
79.79 ± 33.56 |
147.74 ± 30.58***
|
|
Niacin (%) |
50.78 ± 18.36 |
89.51 ± 17.10***
|
48.75 ± 18.25 |
83.01 ± 28.15*
|
51.27 ± 18.61 |
91.09 ± 13.28***
|
|
Vitamin B6 (%) |
78.91 ± 33.06 |
204.44 ± 38.73***
|
81.05 ± 24.51 |
176.58 ± 27.82***
|
48.39 ± 35.09 |
211.21 ± 38.23***
|
|
Pantothenic acid§ (%) |
73.55 ± 34.22 |
122.32 ± 22.78***
|
91.14 ± 59.58 |
106.11 ± 33.64 |
69.27 ± 24.05 |
126.26 ± 17.77***
|
|
Folate (%) |
80.95 ± 32.02 |
119.54 ± 23.76***
|
82.72 ± 41.98 |
103.27 ± 25.69 |
80.52 ± 29.82 |
123.49 ± 21.83***
|
|
Vitamin D§ (%) |
39.31 ± 52.95 |
226.86 ± 24.63***
|
82.56 ± 106.62 |
240.59 ± 40.20**
|
28.79 ± 19.92 |
223.52 ± 18.46***
|
|
Calcium (%) |
47.57 ± 33.59 |
100.64 ± 18.25***
|
61.71 ± 66.51 |
86.23 ± 26.24 |
44.13 ± 19.10 |
104.15 ± 14.10***
|
|
Magnesium (%) |
22.17 ± 11.95 |
23.48 ± 12.03 |
28.26 ± 18.84 |
31.30 ± 20.27 |
20.68 ± 9.39 |
21.58 ± 8.40 |
|
Iron (%) |
70.99 ± 32.64 |
141.92 ± 37.14***
|
74.05 ± 36.30 |
120.90 ± 34.39*
|
70.25 ± 32.18 |
147.03 ± 36.39***
|
|
Zinc (%) |
64.16 ± 29.18 |
165.08 ± 48.16***
|
74.02 ± 32.05 |
142.38 ± 24.47**
|
61.76 ± 28.39 |
170.61 ± 24.04***
|
|
Leucine (%) |
96.59 ± 57.47 |
159.55 ± 36.33***
|
119.18 ± 96.73 |
158.75 ± 68.02 |
91.09 ± 43.47 |
159.75 ± 24.93***
|
Changes in physical measurements
The changes in physical measurements before and after the intake of protein nutrient drinks are presented in
Table 6. In a study on intervention, the compliance to the intake of the provided food is generally set to ≥ 80%. As a result, the weight and height of all 90 participants showed a significant increase after the intake of the nutrient drink (p < 0.001) with a significant increase in BMI (p < 0.01). The participants with ≥ 80% compliance (n = 46) also showed a significant increase in weight and height but no significant difference in BMI after the intake of the nutrient drink.
Table 6Change of anthropometric measurement during 12-week study period
Table 6
|
Variable |
Intervention (n = 90) |
≥ 80% compliance (n = 46) |
|
Before |
After |
Before |
After |
|
Weight (kg) |
36.77 ± 14.55 |
38.13 ± 14.57***
|
39.18 ± 14.83 |
40.46 ± 14.69**
|
|
Height (cm) |
144.04 ± 15.50 |
145.28 ± 15.16***
|
144.46 ± 14.99 |
146.20 ± 14.34***
|
|
Body mass index (kg/m2) |
17.07 ± 4.36 |
17.41 ± 4.34**
|
18.18 ± 4.39 |
18.35 ± 4.39 |
Changes in body composition
The changes in body composition before and after the intake of protein nutrient drinks are presented in
Table 7. The results of skeletal muscle mass, fat free mass, and body fat mass are shown. Although 30 participants had agreed to the InBody measurements to examine the muscle status, the results could be obtained for only seven participants owing to absence on the day of measurements or serious health issues, such as edema. The body composition after the intake of the protein nutrient drink showed the following changes: increase in skeletal muscle mass and fat free mass by 11.57%, and 10.67%, respectively, and a decrease in body fat mass by 0.68%. However, between the times before and after the intake of the nutrient drink, these changes were not statistically significant.
Table 7Muscle health and physical performance changes during 12-week study period
Table 7
|
Variable |
Disabled (n = 7) |
|
Before |
After |
Change ratio (%)*
|
|
Skeletal muscle mass (kg) |
15.99 ± 5.02 |
17.84 ± 4.52 |
▲11.57 |
|
Fat free mass (kg) |
30.54 ± 8.42 |
33.80 ± 7.33 |
▲10.67 |
|
Body fat mass (kg) |
10.31 ± 7.60 |
10.24 ± 5.40 |
▼0.68 |
DISCUSSION
This study was conducted to investigate the current status of dietary intake in children and adolescents with brain lesions residing in Incheon, South Korea, with an aim to analyze the problems in their diet and identify the effects of the 12-week intake of a protein nutrient drink (containing multiple nutrients from proteins, vitamin D, to calcium, minerals and vitamin.) on the improvements of nutrient and muscle statuses via continuous high-protein nutrient supply.
Most studies on children with brain lesions in South Korea had been conducted on children in special-education schools or care centers to restrict the age of the children to ≤ 12 years. The studies had also focused more on the current nutrient intake than on nutrient management [
24]. The children at care centers received nutrient management in part as they were provided with one meal a day upon their visit to the center. However, in this study the age range was extended to include adolescents who have greater demand for nutrients due to rapid physical development, which was in contrast to most previous studies conducted in South Korea [
25].
The participants usually receive at least one meal a day during the visit to the institution. However, the participants were not allowed to visit the institution as this study was conducted after the outbreak of COVID-19 and therefore the current nutrient intake at home was examined instead.
The age range of the participants in this study was 8–19 years, and 93.3% of the participants had brain lesions or brain lesion combined with other disorders. For assessing physical characteristics, height, weight, and BMI of the participants in this study were compared with the standard values in the growth chart for children and adolescents. The result showed that, except for the weight and BMI of females aged 8–11 years, the level of physical development was low across all age groups. The survey of dietary intake to analyze the current nutrient intake of the participants showed that, except vitamin B
1, the levels of nutrients in all age groups did not reach 100% of the recommended levels. Notably, the intake of proteins as essential nutrients in the growth and the increase in muscle mass during childhood and adolescence [
13] and in the production of antibodies for immunity, enzymes and hormones, fell below approximately 80% to imply inadequate intake.
The levels of calcium and vitamin D intake with a critical role in muscle health besides proteins were analyzed, and the results showed that the intake of calcium was approximately < 60% of the recommended intake and the intake of vitamin D was approximately < 50% of the recommended intake, with the exception of participants aged 8–11 years at week 0 implying more inadequacy than the intake of proteins. Calcium is involved in muscle contraction and relaxation [
26] and vitamin D facilitates protein synthesis by promoting muscle synthesis and calcium absorption to increase the intracellular ATP concentration [
27]. Thus, besides proteins, the intake of vitamin D and calcium are important for the muscle health of children with brain lesions. In addition, the intake of magnesium was approximately < 40% of the RNI to indicate the lowest level of intake. Magnesium plays a role as a component in bones and teeth and as a cofactor in enzymes to perform crucial functions across various biochemical and physiological processes from cell membrane stability, neurotransmission, and synthesis of fats, proteins, and nucleic acids [
28]. Magnesium is also an essential nutrient constituting bones alongside calcium indicating that it is highly important during the growth of children in relation to skeletal health. The inadequate intake of such a variety of nutrients could cause poor growth and reduced muscle strength so that the affected individuals spend most of their time in bed. The reduced immunity could also induce various other diseases. Thus, adequate nutrient supply is indispensable for a healthy life.
Among the 90 participants in this study, 46 completed the 12-week supplementation with a compliance of greater than 80%, and their levels of nutrient intake except for the intake of magnesium showed a significant increase with a consequent effect on weight gain. The changes in physical characteristics in accordance with the compliance to the supplementation showed a significant increase in height, weight, and BMI after the intake of protein nutrient drink across all 90 participants. In addition, 35 participants even with 50% to < 80% compliance also showed a significant increase in height, weight, and BMI (data not shown) suggesting that continuous supply of nutrients could improve the nutrient status in children with brain lesions.
In line with this, numerous studies have been conducted in overseas countries investigating the nutrient status and physical characteristics per disability (cerebral palsy, developmental disorder) in accordance with established guidelines of dietary management [
21]. In South Korea, however, the children and adolescents with disabilities as well as those with brain lesions are yet to be assessed for nutrient status, while there is no guideline for nutrient intake. Thus, it is necessary that a guideline of nutrient supply and health management be developed based on the findings in studies investigating the nutrient status and physical characteristics of children and adolescents with disabilities in South Korea.
There were several limitations in this study. First, only approximately half (n = 46) of the participants in this study showed 80% or higher compliance to the intake of protein nutrient drink as children with brain lesions often face difficulties in drinking or could not finish the amount provided. Second, the InBody device was used to measure the muscle mass to estimate muscle health, but, because of the involuntary movements or rigidity of a body part in certain children with brain lesions, accurate measurements were difficult to obtain. Third, we are aware of the failure to use a control group (same population with no supplementation) can make it impossible to draw meaningful conclusion, but we did not want to expose them to the potential harms of non-treatment.
Nevertheless, we suggest that reinforced education and management of compliance to supplementation should be ensured for the participants in order to receive an adequate nutrient supply. Furthermore, patient-centered tools of assessing nutrient status and body composition for children and adolescents with disabilities should be developed to ensure better patient care.
CONCLUSION
Through a survey of dietary intake and physical measurements for children with brain lesions in this study, the levels of growth and nutrient intake were found to be significantly lower in these children than in healthy children. In addition, compared to children aged 8–11 years, those aged 12–19 years showed lower levels of proteins, leucine, and vitamin D as part of nutrient intake assessment, while the requirement of these nutrients that promote muscle growth was higher in children aged 12–19 years as they undergo adolescence with distinctly increased skeletal growth [
29]. Therefore, the level of calorie intake as well as nutrient intake for proteins, calcium, and vitamin D required for muscle and skeletal growth in children and adolescents should be increased. The effects of a 12-week intake of protein nutrient drink with respect to the level of nutrient intake, nutrient status, and muscle status before and after the intake yielded significant improvements in nutrient status as well as physical and muscle health.
While we successfully showed that the supplementation of nutrients through beverages is predicted to help improve the nutrient status and health of children and adolescents with brain lesions, further studies regarding the development of specific guidelines on nutrient intake for this population should be urgently conducted. The findings in this study are expected to serve as basic data in the development of guidelines of nutrient intake for children with severe disabilities in South Korea.
NOTES
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Park YK, Kim H, Lee H, Kim MN, Park SJ, Park HS.
Investigation: An X, Yoon H, Park HS.
Methodology: Park YK, Kin HJ, Lee H, Kim MN, Park HS.
Project administration: Park SJ, Park YK.
Software: Yoon H, An X.
Supervision: Park YK, Park SJ, Go GW.
Validation: Park YK, Go GW.
Writing - original draft: Yoon H, Park HS.
Writing - review & editing: Park YK, Go GW.
REFERENCES
- 1. Bax M. Eating is important. Dev Med Child Neurol 1989;31:285-286.
- 2. Blackman JA. Medical aspects of developmental disabilities in children birth to three. Gaithersburg (MD): Aspen Publishers; 1990.
- 3. O’Brien S, Repp AC, Williams GE, Christophersen ER. Pediatric feeding disorders. Behav Modif 1991;15:394-418.
- 4. Fung EB, Samson-Fang L, Stallings VA, Conaway M, Liptak G, Henderson RC, Worley G, O’Donnell M, Calvert R, Rosenbaum P, Chumlea W, Stevenson RD. Feeding dysfunction is associated with poor growth and health status in children with cerebral palsy. J Am Diet Assoc 2002;102:361-373.
- 5. Chung ON. The effects of behavioral intervention procedures on eating problems of pervasive developmental disorders [dissertation]. Daegu: Daegu University; 1996.
- 6. Kim EK, An SY, Kim EM, Huh KJ, Kim EK. A comparison of the eating habits and eating behaviors of disabled and non-disabled children. Korean J Community Nutr 2003;8:840-855.
- 7. Murphy SM, Caretto VC. Sensory aspects of feeding. In Lewman DK, Murphy SM, eds, ddThe educator’s guide to feeding children with disabilities. Baltimore (MD): Paul H. Brookes Publishing Co., Inc; 1999, pp 111-126.
- 8. Stevenson RD, Hayes RP, Cater LV, Blackman JA. Clinical correlates of linear growth in children with cerebral palsy. Dev Med Child Neurol 1994;36:135-142.
- 9. Stallings VA, Charney EB, Davies JC, Cronk CE. Nutrition-related growth failure of children with quadriplegic cerebral palsy. Dev Med Child Neurol 1993;35:126-138.
- 10. Shapiro BK, Green P, Krick J, Allen D, Capute AJ. Growth of severely impaired children: neurological versus nutritional factors. Dev Med Child Neurol 1986;28:729-733.
- 11. Rempel GR, Colwell SO, Nelson RP. Growth in children with cerebral palsy fed via gastrostomy. Pediatrics 1988;82:857-862.
- 12. Sanders KD, Cox K, Cannon R, Blanchard D, Pitcher J, Papathakis P, Varella L, Maughan R. Growth response to enteral feeding by children with cerebral palsy. JPEN J Parenter Enteral Nutr 1990;14:23-26.
- 13. Bell KL, Samson-Fang L. Nutritional management of children with cerebral palsy. Eur J Clin Nutr 2013;67(Suppl 2):S13-S16.
- 14. EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific opinion on dietary reference values for protein. EFSA J 2012;10:2557.
- 15. Korea Health Industry Development Institute. National nutrition statistics nutrient intake [Internet]. 2018. cited 2020 August 5. Available from https://www.khidi.or.kr/kps/dhraStat/result5?menuId=MENU01657&gubun=&year=
- 16. Jang YK, Park HR, Byun KW, Lee BK, Kwon JS. Basic nutrition. 4th ed. Paju: Kyomunsa; 2017.
- 17. Duncan B, Barton LL, Lloyd J, Marks-Katz M. Dietary considerations in osteopenia in tube-fed nonambulatory children with cerebral palsy. Clin Pediatr (Phila) 1999;38:133-137.
- 18. Jones M, Campbell KA, Duggan C, Young G, Bousvaros A, Higgins L, Mullen E. Multiple micronutrient deficiencies in a child fed an elemental formula. J Pediatr Gastroenterol Nutr 2001;33:602-605.
- 19. Saito Y, Hashimoto T, Sasaki M, Hanaoka S, Sugai K. Effect of selenium deficiency on cardiac function of individuals with severe disabilities under long-term tube feeding. Dev Med Child Neurol 1998;40:743-748.
- 20. Shim JE, Yoon JH, Kim KJ, Paik HY. Association between picky eating behaviors and growth in preschool children. J Nutr Health 2013;46:418-426.
- 21. Ekvall SW, Ekvall VK. Pediatric and adult nutrition in chronic diseases, developmental disabilities, and hereditary metabolic disorders: prevention, assessment, and treatment. Oxford: Oxford University Press; 2017.
- 22. Lee JW, Lee HS, Chang NS, Kim JM. The relationship between nutrition knowledge scores and dietary behavior, dietary intakes and anthropometric parameters among primary school children participating in a nutrition education program. J Korean Nutr 2009;42:338-349.
- 23. Ahn HY, Im SB, Hong KJ, Hur MH. The effects of a multi agent obesity control program in obese school children. J Korean Acad Nurs 2007;37:105-113.
- 24. Kim HJ, Choi HN, Yim JE. Food habits, dietary intake, and body composition in children with cerebral palsy. Clin Nutr Res 2018;7:266-275.
- 25. Stang J, Story MT. Guidelines for adolescent nutrition services. Minneapolis (MN): Center for Leadership, Education and Training in Maternal and Child Nutrition, Division of Epidemiology and Community Health; 2005.
- 26. Szent-Györgyi AG. Calcium regulation of muscle contraction. Biophys J 1975;15:707-723.
- 27. Halfon M, Phan O, Teta D. Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. BioMed Res Int 2015;2015:953241.
- 28. Swaminathan R. Magnesium metabolism and its disorders. Clin Biochem Rev 2003;24:47-66.
- 29. Kim YK, Nam HW, Park YS, Myeong CO, Lee KW. Nutrition across the life span. Seoul: Shinkwang Publishers; 2001, pp 188-189.
 , Hyoung su Park1
, Hyoung su Park1 , Xiangxue An1
, Xiangxue An1 , Seok Jun Park1
, Seok Jun Park1 , Gwang Woong Go2
, Gwang Woong Go2 , Hyunjung Kim3
, Hyunjung Kim3 , Hyesoon Lee4
, Hyesoon Lee4 , Mee Na Kim5
, Mee Na Kim5 , Yoo Kyoung Park6
, Yoo Kyoung Park6