ABSTRACT
-
Background
Vitamin D participates in the biological function of the innate and adaptive immune system and inflammation. We aim to specify the effectiveness of the vitamin D supplementation on the side effects BioNTech, Pfizer vaccination, and immunoglobulin G response against severe acute respiratory syndrome coronavirus 2 in subjects tested positive for coronavirus disease 2019 (COVID-19).
-
Methods
In this multi-center randomized clinical trial, 498 people tested positive for COVID-19 were divided into 2 groups, receiving vitamin D capsules or a placebo (1 capsule daily, each containing 600 IU of vitamin D) over 14–16 weeks. Anthropometric indices and biochemical parameters were measured before and after the second dose of vaccination.
-
Result
Fourteen to 16 weeks after supplementation, the intervention group had an immunoglobulin G (IgG) increase of 10.89 ± 1.2 g/L, while the control group had 8.89 ± 1.3 g/L, and the difference was significant between both groups (p = 0.001). After the second dose of vaccination, the supplement group significantly increased their 25-hydroxy vitamin D from initially 28.73 ± 15.6 ng/mL and increased to 46.48 ± 27.2 ng/mL, and the difference between them was significant. Those with a higher body mass index (BMI) had the most of symptoms, and the difference of side effects according to BMI level was significantly different. In 8 weeks after supplementation obese participants had the lowest IgG levels than overweight or normal subjects. The proportion of all types of side effects on the second dose was significantly diminished compared with the first dose in the intervention group.
-
Conclusion
Supplementation of 600 IU of vitamin D3 can reduce post-vaccination side effects and increase IgG levels in participants who received BioNTech, Pfizer vaccine.
-
Trial Registration
-
Keywords: Vitamin D; Immunoglobulin G; COVID-19 vaccine; Side effects
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a coronavirus known to infect humans discovered in China in 2019 as the source of an unknown outbreak [
1]. Since there was a shortage of vaccines or other treatment methods, several preventive techniques have been adopted to stop the pandemic, including quarantine, the use of face masks, and the use of antiseptics [
2]. The Food and Drug Administration (FDA) ratified Pfizer-BioNTech vaccine on December 11, 2020 [
3]. As the Pfizer-BioNTech coronavirus disease 2019 (COVID-19) vaccine tried to combat SARS-CoV-2, there was a need to conduct studies on the side effects of the vaccine and its effectiveness, and in this way, thereby increasing the acceptance rate of the people to vaccination [
4]. The intensity of side effects varies greatly depending on the recipient’s body mass index (BMI) and nutritional status [
5].
There is evidence that those who showed fewer side effects after vaccination has higher antibody titers, which may indicate better protection. Also, subjects who had higher BMI showed more side effects after vaccination [
6]. As obesity is a considerable predictor of mortality in COVID-19 patients, it is important to investigate the relationship between anthropometric indices and COVID-19 vaccination [
7].
Supplementation with micronutrients appears as a necessary measure to ameliorate the immune system and prevent adverse side effects. Therefore, vitamin D has attracted a lot of attention [
8,
9,
10]. Vitamin D deficiency is considered a main public health problem [
11]. Other researchers showed that hypocalcemia and vitamin D deficiency increase the risk of respiratory infections [
12]. The role of vitamin D as an immunomodulator has been proven in previous studies [
13]. Christianto and Smarandache [
14], showed that vitamin D3 (Vit-D3) has several beneficial effects in coping with a virus, including boosting the immune system to disassemble the viral protein glycoprotein. Thus, this study aimed to peruse the role of Vit-D3 intervention on the side effects of BioNTech, Pfizer vaccination, and immunoglobulin G (IgG) response against SARS-CoV-2 in subjects tested positive for COVID-19.
MATERIALS AND METHODS
Participants and study design
It is a multi-centered, 1:1 randomized clinical trial. Participants who were tested positive for COVID-19 and their serum 25-hydroxy vitamin D (25[OH]D) below 30 ng/mL were included. The exclusion were those who did not take the vaccine or who take one dose of the vaccine and did not come back for another dose, those who in the last 6 months received vitamin or mineral supplementation, those with chronic conditions such as diabetes, hypertension, and heart disease, unwillingness to continue the study protocol, lactating women and pregnant women.
The Ethics Committee of Garmian Polytechnic University, Kalar Technical College approved the study (No. KTC20230105). It was also registered in ClinicalTrials.gov (
NCT05851313) on December 5, 2023. All methods were performed following relevant guidelines and regulations. Verbal and written informed consent was obtained from all the participants before the study. This study was conducted based on the Declaration of Helsinki.
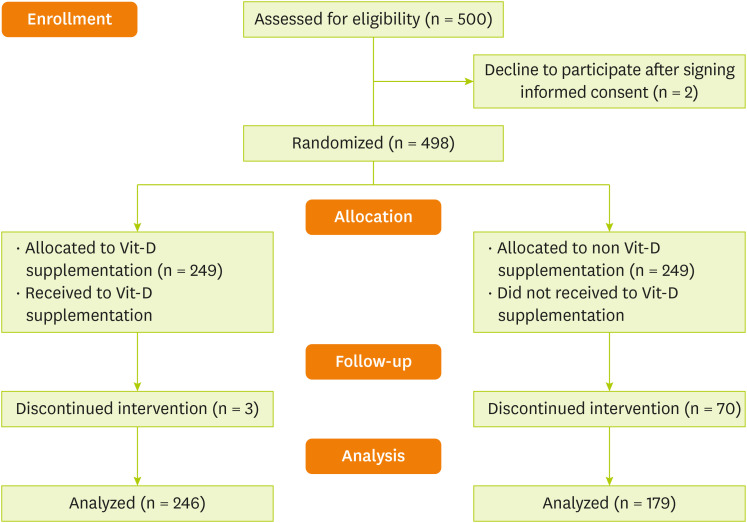
The sample size was computed relying on a previous study [
15] considering the proportion of fever ≥ 38°C in normal weight as 9.8% and in obese people as 1.8%, study power of 80%, and confidence level of 95%. For each group, the estimated sample was 183 subjects. Considering the attrition rate of 25% during the follow-up period, the sample size reached 248 subjects in each group. This study was a double-blinded clinical trial. A total of 500 patients met the inclusion criteria for our study and 2 participants declined to participate after signing informed consent (
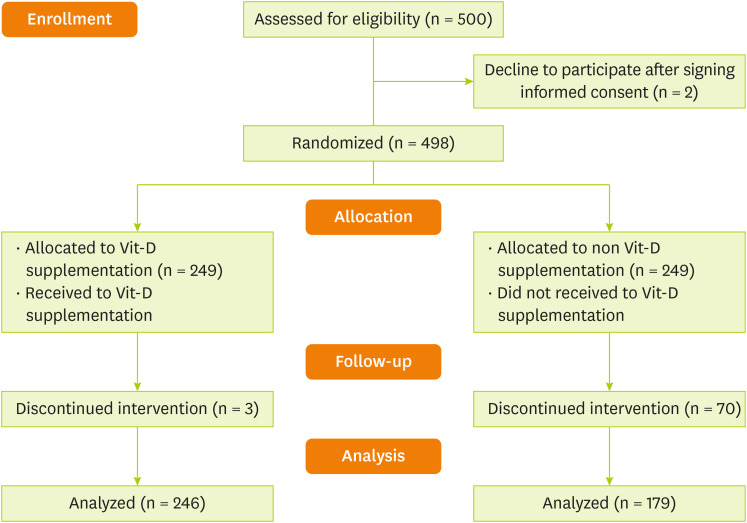
Figure 1). Permuted block randomization method was used to randomly assign patients to study groups. For the sample size (n = 500), we generated 84 block sizes of 6. The study groups were coded as A (for the intervention group) and B (for the placebo group).
Figure 1
Patients flow diagram.
Vit-D, vitamin D.
The intervention group received 600 IU of vitamin D supplements every day, one hour after breakfast for 14–16 weeks. The control group took a placebo prepared with the same shape and size of supplements. The supplement was given to the patients by someone other than the researcher, to ensure that the researchers did not know which group receiving the supplement or placebo (given the double-blindness of the study). Also, in this study, the person who did the data analysis did not aware of randomization. At first, a 3-day recall food questionnaire was taken from 2 groups. From the day, the subjects were tested for positive, they were asked to start supplementation for 4 weeks and return after that to take the first dose of the vaccine. Subjects were asked to continue to use supplements after the first vaccination. After 6 to 8 weeks, patients came back for another vaccination dose and were again asked to use the supplement for 4 weeks again, after that subjects were asked to come for a blood test.
Physical activity
The rate of the subject’s physical activity was evaluated by using the validated International Physical Activity Questionnaire (IPAQ). Based on metabolic equivalents (METs), recorded quantities were displayed and separated into 3 types (light: < 600, moderate: 600–3,000, and high > 3,000 MET-min/week) [
16].
Laboratory tests to detect SARS-CoV-2 infection were performed by reverse transcription polymerase chain reaction (RT-PCR). Also, for viral RNA extraction, a nasopharyngeal swab sample was collected. In the next step, total RNA was automatically extracted in 45 minutes by using the Qiagen EZ1 Advanced XL system (Qiagen, Hilden, Germany). The presence of SARS-CoV-2 was then diagnosed by real-time RT-PCR amplification of SARS-CoV-2 open reading frame 1 ab (ORF1ab) and envelope (E) gene fragments by using Power Chek SARS-CoV-2 Real-Time PCR kit (Kogenebiotech, Seoul, Korea).
Anthropometric indices
Body weight was determined to the nearest 0.1 kg using a bioimpedance analyzer (Inbody 770; Inbody Co, Seoul, Korea). Height was measured using a BSM 370 (Biospace Co., Seoul, Korea). To calculate a person’s BMI, multiply their weight in kilograms by the square of their height (in meters). According to the guideline by the Centers for Disease Control and Prevention (CDC), a BMI of less than 18.5 kg/m
2 identify as underweight, 18.5–24.9 kg/m
2 is a normal or healthy weight and 25.0–29.9 kg/m
2 is overweight, and 30.0 kg/m
2 or higher is obese [
17].
We divided the side effects of vaccination into 5 categories; gastrointestinal symptoms involving fever and chills, pain (involving local pain, myalgia, and headache), local bruising and local reactions, and no symptoms. Patients were asked to specify in a questionnaire prepared by the researchers which of these side effects they had between the 2 doses and also after the second dose.
Clinical and laboratory results
The blood test was taken twice from subjects in the morning after a 12-hour fast in the 4 weeks after the first dose of vaccine and in the 6–8 weeks after the second vaccination. Blood samples were collected by venipuncture from the antecubital vein by vacuum tubes. To obtain blood serum, all blood sample was centrifuged at 2,500 rpm for 10 minutes. Laboratory tests were carried out on the same day without reserving the serum of patients and according to the ready prediction in the laboratory using ELx800 (BioTek Instruments, Inc., Winooski, VT, USA) ELISA test and measuring the IgG titer (g/L). Quantification of 25(OH)D levels (ng/mL) was performed from serum samples using automated immunoassays, Liaison 25(OH) Total Vitamin D Assay DiaSorin Liaison XL (DiaSorin, Gerenzano, Italy).
Statistical analysis
Analyses were done by Stata 14 software (StataCorp LLC, College Station, TX, USA). Frequency (percentage) and mean ± standard deviation were used to demonstrate the demographic and clinical characteristics. Comparison of quantitative or qualitative variables between 2 groups was accomplished by using a t-test and chi-square test, respectively. Two-sample test of proportions was used to contrast the proportion of side effects between two doses of vaccine. The effect of the intervention on the outcomes during the period was also investigated using repeated measures analysis of variance with adjusting the effects of variables such as age, sex, BMI, and smoking. Absolute risk reduction (ARR) or risk difference was calculated as the difference between the proportion of side effects in the placebo and the proportion of side effects in the supplemented vitamin D. ARR measures the direct impact of the intervention on the outcome. In addition, 5 categories of the side effects were summed as another outcome named the frequency of side effects of vaccination (0 = no symptom, 1, 2, 3, and 4 side effects). Linear regression was used to find relationship between the frequency of side effects on the second dose vaccine and the mean BMI of the patients. Also, the relationship between the BMI groups and IgG titer on the first and second dose vaccine between supplemented and placebo groups. Test was done by independent t-test. And paired t-test used to compare the proportion of the side effects of COVID-19 vaccination between the first and second doses by study groups.
RESULTS
Table 1 demonstrates the demographic characteristics of patients within intervention and control groups. Four hundred and twenty-five patients (279 men [65.6%] and 146 women [34.4%]) were enrolled in the study and randomized: 179 placebo group, 246 with vit-D supplementation (
Figure 1). Mean age was 50.78 ± 14.75 years, being 48.56 ± 12.54 years for the placebo group and 52.38 ± 16.0 years for the supplemented group. The mean age was significantly different between the groups (p = 0.008). According to the BMI of study participants, following both vaccine doses, the intervention group significantly had a lower BMI than the placebo group (p = 0.001) and there is a significant difference between 1st and 2nd doses of BMI in both groups (p = 0.006 and p = 0.005, respectively).
Table 1 Baseline characteristics of COVID-19 patients by placebo and supplement groups
Table 1
|
Variable |
Placebo (n = 179) |
Supplement (n = 246) |
p value*
|
|
Age group (yr) |
|
|
< 0.001 |
|
≤ 40 |
58 (32.40) |
72 (29.27) |
|
41–60 |
91 (50.84) |
89 (36.18) |
|
> 60 |
30 (16.76) |
85 (34.55) |
|
Sex |
|
|
< 0.001 |
|
Male |
96 (53.63) |
183 (74.39) |
|
Female |
83 (46.37) |
63 (25.61) |
|
Residency |
|
|
< 0.001 |
|
Urban |
128 (71.51) |
57 (23.17) |
|
Rural |
51 (28.49) |
189 (76.83) |
|
Marital status |
|
|
< 0.001 |
|
Married |
62 (34.64) |
185 (75.20) |
|
Divorced |
88 (49.16) |
36 (14.63) |
|
Single |
29 (16.20) |
25 (10.16) |
|
Socioeconomic status |
|
|
< 0.001 |
|
Low |
97 (54.19) |
56 (22.76) |
|
Normal |
58 (32.40) |
80 (32.52) |
|
Good |
24 (13.41) |
110 (44.72) |
|
Physical activity (MET-min/week) |
|
|
< 0.001 |
|
light |
53 (29.16) |
27 (10.98) |
|
Moderate |
91 (50.84) |
64 (26.02) |
|
High |
35 (19.55) |
155 (63.01) |
|
Smoking status |
|
|
0.005 |
|
No |
129 (72.07) |
145 (58.94) |
|
Yes |
50 (27.93) |
101 (41.06) |
|
Age (yr) |
48.56 ± 12.54 |
52.38 ± 16.00 |
< 0.001 |
|
BMI (kg/m2) |
|
|
|
|
1st dose |
28.62 ± 4.88 |
24.67 ± 4.49 |
< 0.001 |
|
2nd dose |
28.80 ± 4.71 |
24.85 ± 4.31 |
< 0.001 |
|
p value†
|
0.006 |
0.005 |
- |
Table 2 shows the association between supplemented vitamin D and IgG antibody and 25(OH)D concentrations in serum of COVID-19 patients. In eight weeks after the enrollment of participants and 4 weeks after the first dose, the IgG level of supplemented participants was 2.01 ± 1.1 g/L, while the control group was 1.96 ± 1.1 g/L, and it was not significant (p = 0.562). In 4–6 weeks after the second dose, which corresponds to 14–16 weeks after the intervention, the supplemented participants had an IgG increase of 10.89 ± 1.2 g/L, while the control group had 8.89 ± 1.3 g/L and this difference was significant between both groups (p = 0.001).
Table 2 Association of supplemented vitamin D with IgG antibody and 25(OH)D concentrations in serum among COVID-19 patients
Table 2
|
Variable |
Placebo |
Supplement |
p value†
|
|
IgG (g/L) |
|
|
|
|
First dose |
1.96 ± 1.1 |
2.01 ± 1.1 |
0.562 |
|
Second dose |
8.89 ± 1.3 |
10.89 ± 1.2 |
0.001 |
|
p value |
0.001 |
0.001 |
- |
|
25(OH)D (ng/mL) |
|
|
|
|
First dose |
26.09 ± 14.9 |
28.73 ± 15.6 |
0.245 |
|
Second dose |
21.25 ± 21.3 |
46.48 ± 27.2 |
0.001 |
|
p value*
|
0.001 |
0.001 |
- |
As for 25(OH)D, after the first dose of vaccination, there was no difference between the 25(OH)D levels of participants between the intervention and placebo groups. However, after the second dose of vaccination, the supplement group significantly increased their 25(OH) D levels from initially 28.73 ± 1.6 ng/mL and increased to 46.48 ± 27.2 ng/mL, the difference between them was significant (p = 0.001). The control group, unlike the supplement group after the second dose, decreased their 25(OH)D from 26.09 ± 14.9 ng/mL to 21.25 ± 21.3 ng/mL (p = 0.001).
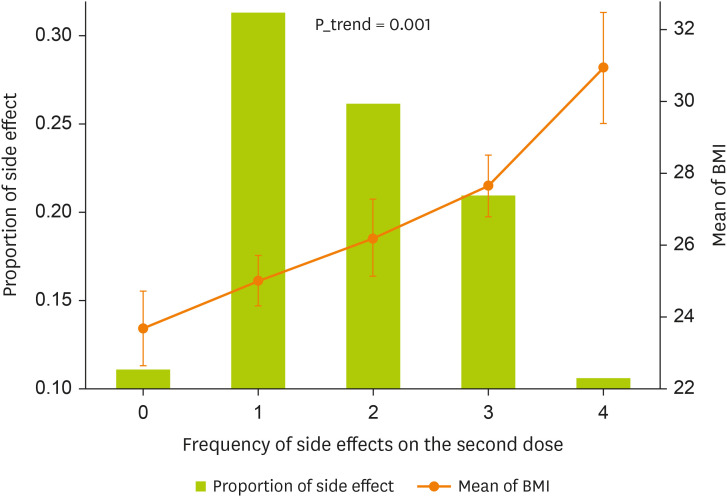
A comparison of the side effects of Pfizer vaccination by weight groups among participants was shown in
Figure 2 and
Table 3. The number of side effects of the Pfizer vaccination increases with BMI increases. The majority of subjects with normal weight (68.1%), had no adverse reactions to the vaccination while those with overweight (27.7%) and obese (4.2%) had adverse reactions.
Figure 2
The relationship between the frequency of side effects on the second dose vaccine and the mean BMI of the patients. Test was done by linear regression.
BMI, body mass index.
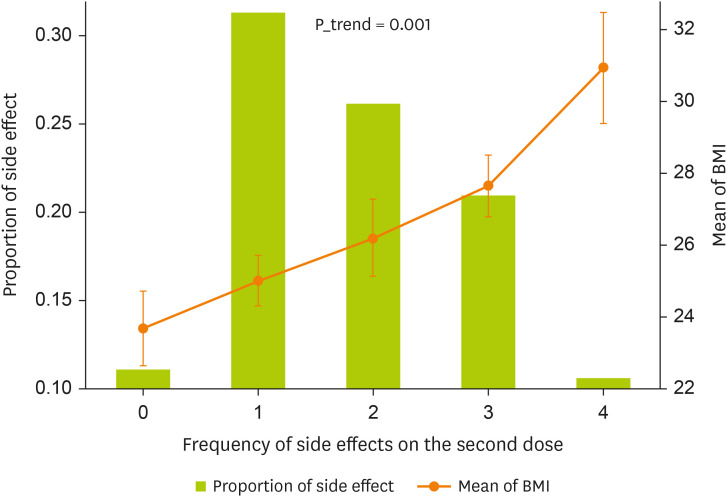
Table 3 Comparison of Pfizer vaccine side effects by weight groups among COVID-19 patients
Table 3
|
Frequency of side effects on the 2nd dose |
BMI (kg/m2) |
|
Normal (18.50–24.99) |
Overweight (25.0–29.99) |
Obese (> 30.0) |
Mean ± SD |
|
0 |
32 (68.1) |
13 (27.7) |
2 (4.2) |
23.70 ± 3.6 |
|
1 |
80 (60.2) |
43 (32.3) |
10 (7.5) |
25.0 ± 4.1 |
|
2 |
60 (54.1) |
32 (28.8) |
19 (17.1) |
26.19 ± 5.7 |
|
3 |
24 (27.0) |
43 (48.3) |
22 (24.7) |
27.63 ± 4.1 |
|
4 |
4 (8.9) |
20 (44.4) |
21 (46.7) |
30.92 ± 5.3 |
|
p value |
0.001*
|
0.001†
|
Also, of those who were obese 46.7% had the most adverse reactions to the vaccination while only 8.9% of normal weight had adverse reactions to the vaccination. In other words, those with a BMI of 23.70 ± 3.6 kg/m2 had no side effects, while those with 30.92 ± 5.3 kg/m2 had the most symptoms, and the difference between BMI of side effects was statically significant (p = 0.001).
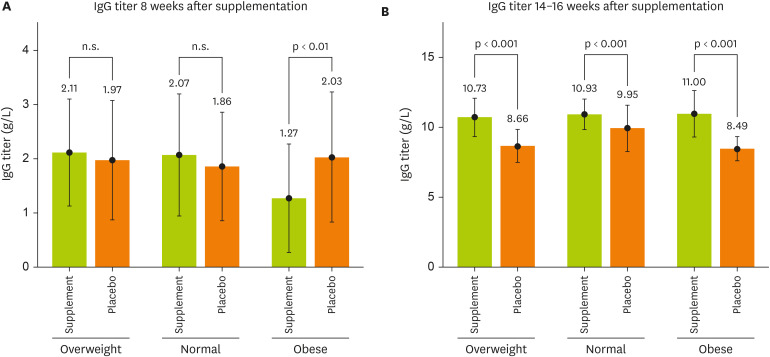
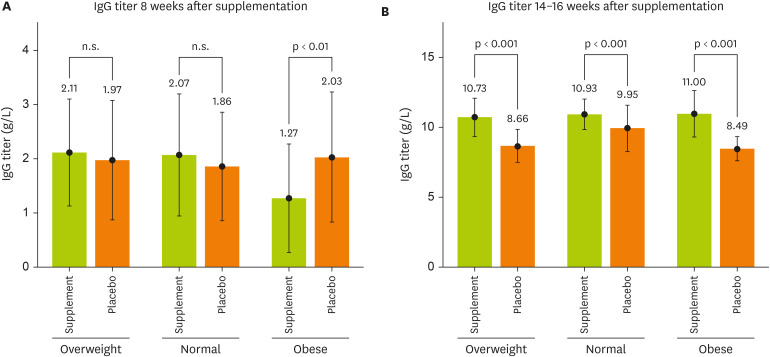
Figure 3 illustrated the association among the BMI groups and IgG titer on the 1st and 2nd dose vaccine between supplemented and placebo groups. In eight weeks after supplementation, there was no significant difference between the overweight and normal groups. However, in the obese group, the IgG of the participants who received the placebo was 2.03 g/L while the supplement group was 1.27 g/L, respectively, (p < 0.001;
Figure 3A). In the 14–16 weeks after receiving the supplement, a significant difference was observed between the supplement and placebo groups of the normal, overweight, and obese groups, with the overweight participants taking the supplement having an IgG titer of 10.73 g/L while the placebo group had an IgG of 8.66 g/L, respectively (p < 0.001). Normal weight group, the supplement users had an IgG titer of 10.93 g/L while the placebo group had 9.95 g/L, respectively (p < 0.001). As for the obese group, the supplemented IgG titer was 11.00 g/L while the IgG titer in the placebo group was 8.49 g/L, respectively (p < 0.001;
Figure 3B).
Figure 3
The relationship between the BMI groups and IgG titer on the first and second dose vaccine between supplemented and placebo groups. Test was done by independent t-test.
BMI, body mass index; IgG, immunoglobulin G.
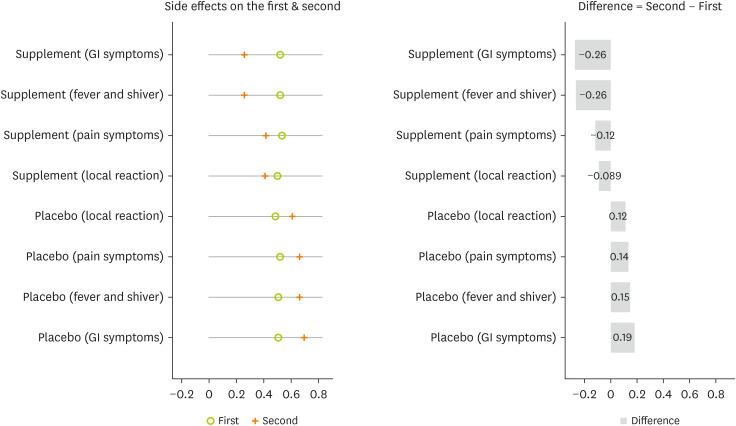
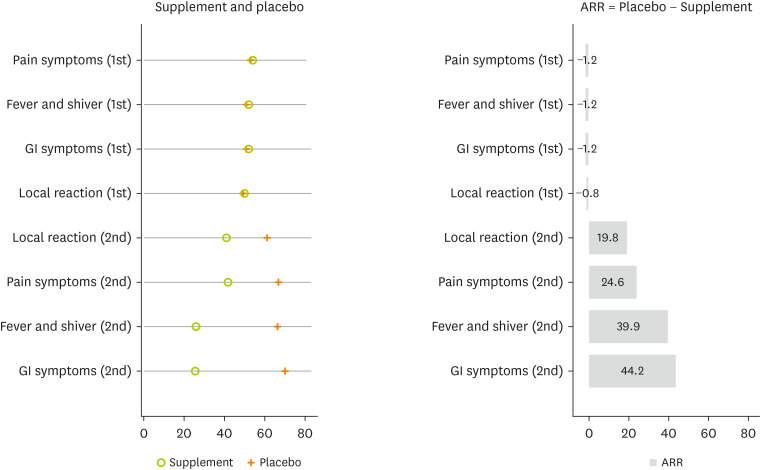
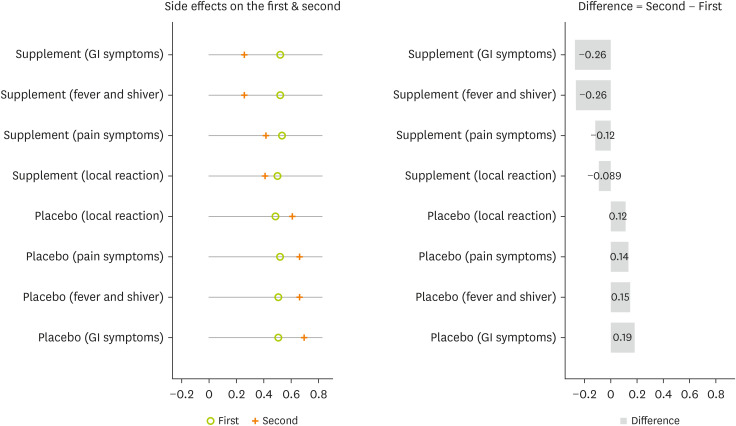
A comparison of the proportion of side effects in the second and first doses of the vaccine shows that supplementation is more effective in reducing the proportion of side effects. The proportion of all types of side effects on the second dose was significantly diminished compared with the first dose in the intervention group. Inversely, the proportion of all types of side effects on the second dose was significantly increased compared with the first dose in the placebo group (
Figure 4).
Figure 4
Comparison the proportion of COVID-19 vaccine side effects between the first and second doses by study groups. Test was done by paired t-test.
COVID-19, coronavirus disease 2019; GI, gastrointestinal.
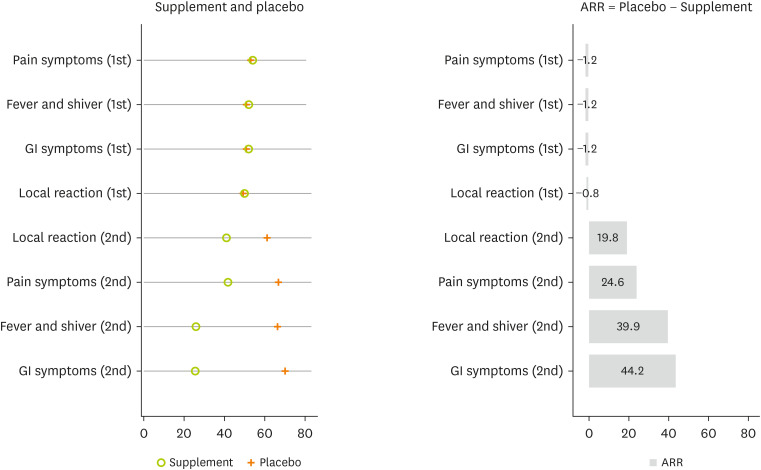
Compared with the placebo group, the supplemented group was associated with a reduction in side effects on the second dose. For example, the proportion of gastrointestinal symptoms on the second dose were 25.6% (20.5%–31.4%) for the supplemented group and 69.8% (62.7%–76.1%) for the placebo group, with an absolute risk reduction of 44.2% (
Figure 5).
Figure 5
The proportion of side effects reduction by supplemented vitamin D using ARR. ARR calculated as: Proportion of Side Effect in the Placebo − Proportion of Side Effect in the Supplemented Vitamin D.
ARR, absolute risk reduction; GI, gastrointestinal.
DISCUSSION
To the best of our knowledge, this is the first study in a Kurdish population to examine the role of BMI and Vit-D supplementation on the side effects of BioNTech, Pfizer vaccination, and immunoglobulin G response against SARS-CoV-2. This study showed that the Vit-D3 supplementation for 14–16 weeks IgG and 25(OH)D levels significantly increased and side effects decreased after the second vaccination compared to the first vaccination. Participants who had normal weight in the intervention group had higher IgG levels and a significantly lower incidence of side effects than obese or overweight participants. While nutritional supplements have been considered as an excellent option to reduce the side effects of COVID-19 vaccination, preliminary research for the evidence has not been in this regard, and the recommendation to exceed the Recommended Dietary Allowances (RDA) without solid data and rely on hypotheses can hurt people in the COVID-19 pandemic. Vitamin D deficiency is very common in COVID-19 patients [
18]. In this context, previous reviews have described how vitamin D diminishes the risk of viral infections and strengthens the immune system [
19,
20,
21,
22,
23,
24]. Vitamin D promotes inherent cell-mediated immunity in part through the induction of antimicrobial peptides, including 1,25-dihydroxy vitamin D (1,25[OH]2D), human cathelicidin LL-37 [
25], and defending [
26]. Cathelicidin exhibits direct antibacterial activity against a variety of microorganisms. It includes Gram-positive and Gram-negative bacteria, enveloped and non-enveloped viruses, and fungi [
27]. These peptides from the host immune system can kill foreign pathogens by disrupting their cell membranes and also can thwart the biological activity of endotoxin [
28]. Also, a laboratory study reported that 1,25(OH)2D decreased rotavirus replication in vitro as well as in vivo by a different procedure [
29]. A clinical trial showed that vitamin D3 at 4,000 IU/d reduced dengue virus infection [
30].
Vit-D3 has also received consideration as an immunomodulator [
13]. Calciferol as an active form of vitamin D is a powerful immunomodulator. Many cells of the immune system, containing T lymphocytes, and macrophages have vitamin D receptors. There is considerable documentation that 1-25-dihydroxy Vit-D3 has diverse effects on the immune system. Therefore, it improves immunity and diminishes autoimmunity [
31]. Vit-D3 interacts directly with infection-fighting cells. More than 50 interventional researches have investigated the association between vitamin D and COVID-19 and were registered at ClinicalTrial.gov. Some of them have found promising results.
In our study, anti-SARS-CoV-2 IgG levels were significantly higher in the vitamin D-supplemented group and the supplemented group also had fewer side effects after the 1st and 2nd vaccination dose compared to the placebo group. In consistent with our study, Chel et al. [
32], evaluated the vitamin D status of oral Vit-D3 doses of 600 IU/day, 4,200 IU/week, and 18,000 IU/month, which shows that daily consumption of vitamin D has more impact than weekly or monthly consumption [
32]. Their findings were consistent with the Endocrine Society’s conclusion that serum vitamin D levels of 30 ng/mL (75 nmol/L) or higher can boost the immune system [
33]. Vit-D3 has important effects on both the innate and adaptive immune systems belong its biological functions [
34]. In contrast, Jolliffe et al. [
35] reported that when 25(OH)D was administered to vitamin D-deficient subjects (mean 25[OH]D level of 15.9 ng/mL [39.9 nmol/L]) it cannot show the protective efficacy or immunogenicity on a SARS-CoV-2 vaccination. Chillon et al. [
36] did not find any relationship between 25(OH)D levels and anti-SARS-CoV-2 IgG levels in a healthy participant with vitamin D deficiency (median 25[OH]D levels less than 30 ng/mL [75 nmol/L]) more than four consecutive measurements.
The side effects of COVID-19 vaccination were significantly correlated with vitamin D levels [
37]. Increasing the consumption of each unit of this vitamin directly decreased the incidence of side effects vaccination [
38]. Also, 2 recent meta-analyses: one review of fourteen studies with 999,179 subjects reported that low level of serum 25(OH)D was related to the increased mortality from COVID-19 with an odds ratio of 3.08 [
39]. In this regard, a systematic review of 43 studies also described low vitamin D levels being related to higher mortality from COVID-19 [
40].
Following a previous study in a pilot study of 76 patients who were hospitalized for COVID-19, high-dose of oral calcifediol reduced intensive-care unit admissions. The benefit of calcifediol administration was a concern due to incomplete blinding and inappropriate distribution of confounders [
41]. An randomized controlled trial of Vit-D3 (cholecalciferol, 60,000 IU/day) supplementation in a therapeutic dose of serum 25(OH)D > 50 ng/mL showed significant induction of negative SARS-CoV-2 RNA and decreased fibrinogen levels [
42].
According to our finding, people who were obese were suscetible to risk of the incidence of side effects after vaccination, and this relationship was statistically significant. In malnourished individual who has a weaker immune system, vaccinations can cause fever and other harmful side effects [
43,
44]. Furthermore, subjects of normal weight had significantly higher IgG titers after 8 weeks of supplementation than subjects who were overweight or obese.
Obesity has been associated with T-cell and macrophage dysfunction according to a study [
45]. Decreased cytokine expression in response to infection has been associated with bone marrow macrophage dysfunction and/or monocyte maturation failure, as well as lower Toll-like receptor 2 expression in these cells [
46]. Additionally, changes in the makeup of the microbiome, such as those observed in obesity, may alter the crosstalk between hematopoiesis and the microbiome, aggravating or worsening inflammation or infection in the host [
47]. According to previous research, obesity reduces antibody responses to hepatitis B immunization in adults [
48]. Moreover, an in vitro study discovered that obesity raises circulation levels of pro-inflammatory cytokines and neutrophils, impairing the response to pandemic virus vaccination. The findings from the in vitro study on the influenza virus H1N1 (pH1N1) [
49] provide valuable insights into how obesity can influence immune responses to viral infections. However, the molecular mechanisms underlying this behavior at the B-cell level still remain unknown.
There is a contrast between the result of weight status on the side effects of vaccination. McNeil et al. [
50] researched the side effects of the anthrax vaccine. They reported that arm soreness and arm swelling are common side effects in obese women [
51]. In contrast, Petousis-Harris et al. [
52] reported that subjects with a lower BMI had more pain when injected with the meningococcal B vaccine. Also, Ellulu et al. [
53] performing a study on 2,136 adults in Spain found no significant association between weight status and side effects of the coronavirus vaccination.
Our study has some strengths. This is the first clinical trial to be conducted on a large number of participants. Since the study is a double-blinded clinical trial, the possibility of error is relatively small, and this is the first time that the effects of 2 factors are simultaneously detected in study participants. The length of the study is another strength of the work in that the participants were given supplements for 14–16 weeks. However, the present study had its limitations, therefore, we tried to minimize and control the effects of potential confounders, but unknown confounders may be still overlooked.
CONCLUSION
The findings from this study further reinforced the accumulating evidences that highlights the potential benefits of vitamin D supplementation in improving postvaccination outcomes for COVID-19. By demonstrating the significant role of vitamin D in mitigating side effects and enhancing the immune response to vaccination, this research emphasizes the importance of considering vitamin D status as a crucial factor in optimizing vaccine efficacy and overall public health. However, while these results are promising, it is essential to acknowledge that additional research is necessary to strengthen and expand upon these findings. Future investigations should explore larger and more diverse populations, encompassing various age groups, ethnicities, and health conditions, to ensure the generalizability of the conclusions.
Garmian Polytechnic University
9472
NOTES
-
Funding: This research was supported by Garmian Polytechnic University (grant number: 9472).
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Fateh HL.
Formal analysis: Rezaeian S.
Investigation: Rezaeian S.
Supervision: Moludi J.
Writing - original draft: Fateh HL, Kareem G, Kamari N.
ACKNOWLEDGEMENTS
We would like to thanks each participant and deputy of Garmian Polytechnic University.
REFERENCES
- 1. World Health Organization. Coronavirus disease (COVID-19) situation report-127. Geneva: World Health Organization; 2020.
- 2. World Health Organization. Draft landscape and tracker of COVID-19 candidate vaccines. Geneva: World Health Organization; 2020.
- 3. U.S. Food and Drug Administration. Pfizer-BioNTech COVID-19 vaccine. Silver Spring (MD): U.S. Food and Drug Administration; 2021.
- 4. Ng WH, Tipih T, Makoah NA, Vermeulen JG, Goedhals D, Sempa JB, Burt FJ, Taylor A, Mahalingam S. Comorbidities in SARS-CoV-2 patients: a systematic review and meta-analysis. MBio 2021;12:e03647-20.
- 5. Di Resta C, Ferrari D, Viganò M, Moro M, Sabetta E, Minerva M, Ambrosio A, Locatelli M, Tomaiuolo R. The gender impact assessment among healthcare workers in the SARS-CoV-2 vaccination-an analysis of serological response and side effects. Vaccines (Basel) 2021;9:522.
- 6. Fateh HL, Kamari NM, Ali A, Moludi J, Rezayaeian S. . Association between diet quality and BMI with side effects of Pfizer-BioNTech COVID-19 vaccine and SARS-CoV-2 immunoglobulin G titers. Nutr Food Sci 2023;53:738-751.
- 7. Pellini R, Venuti A, Pimpinelli F, Abril E, Blandino G, Campo F, Conti L, De Virgilio A, De Marco F, Di Domenico EG, Di Bella O, Di Martino S, Ensoli F, Giannarelli D, Mandoj C, Manciocco V, Marchesi P, Mazzola F, Moretto S, Petruzzi G, Petrone F, Pichi B, Pontone M, Zocchi J, Vidiri A, Vujovic B, Piaggio G, Morrone A, Ciliberto G. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine.. EClinicalMedicine 2021;36:100928.
- 8. Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients 2020;12:236.
- 9. Jovic TH, Ali SR, Ibrahim N, Jessop ZM, Tarassoli SP, Dobbs TD, Holford P, Thornton CA, Whitaker IS. Could vitamins help in the fight against COVID-19? Nutrients 2020;12:2550.
- 10. Name JJ, Souza ACR, Vasconcelos AR, Prado PS, Pereira CPM. Zinc, vitamin D and vitamin C: perspectives for COVID-19 with a focus on physical tissue barrier integrity. Front Nutr 2020;7:606398.
- 11. Gray TK, Lowe W, Lester GE. Vitamin D and pregnancy: the maternal-fetal metabolism of vitamin D. Endocr Rev 1981;2:264-274.
- 12. Zisi D, Challa A, Makis A. The association between vitamin D status and infectious diseases of the respiratory system in infancy and childhood. Hormones (Athens) 2019;18:353-363.
- 13. van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol 2005;97:93-101.
- 14. Christianto V, Smarandache F. A review of major role of vitamin D3 in human immune system and its possible use for novel corona virus treatment. EC Microbiol 2020;16.6:10-16.
- 15. Iguacel I, Maldonado AL, Ruiz-Cabello AL, Casaus M, Moreno LA, Martínez-Jarreta B. Association between COVID-19 vaccine side effects and body mass index in Spain. Vaccines (Basel) 2021;9:1321.
- 16. Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, Day NE. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr 2003;6:407-413.
- 17. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63:2985-3023.
- 18. Lau FH, Majumder R, Torabi R, Saeg F, Hoffman R, Cirillo JD, Greiffenstein P. Vitamin D insufficiency is prevalent in severe COVID-19.. medRxiv Forthcoming. 2020.
- 19. Beard JA, Bearden A, Striker R. Vitamin D and the anti-viral state. J Clin Virol 2011;50:194-200.
- 20. Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 2012;76:315-325.
- 21. Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015;7:4240-4270.
- 22. Wei R, Christakos S. Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients 2015;7:8251-8260.
- 23. Lang PO, Aspinall R. Vitamin D status and the host resistance to infections: what it is currently (not) understood. Clin Ther 2017;39:930-945.
- 24. Gruber-Bzura BM. Vitamin D and influenza-prevention or therapy? Int J Mol Sci 2018;19:2419.
- 25. Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol 2009;182:4289-4295.
- 26. Laaksi I. Vitamin D and respiratory infection in adults. Proc Nutr Soc 2012;71:90-97.
- 27. Herr C, Shaykhiev R, Bals R. The role of cathelicidin and defensins in pulmonary inflammatory diseases. Expert Opin Biol Ther 2007;7:1449-1461.
- 28. Agier J, Efenberger M, Brzezińska-Błaszczyk E. Cathelicidin impact on inflammatory cells. Centr Eur J Immunol 2015;40:225-235.
- 29. Zhao Y, Ran Z, Jiang Q, Hu N, Yu B, Zhu L, Shen L, Zhang S, Chen L, Chen H, Jiang J, Chen D. Vitamin D alleviates rotavirus infection through a microrna-155-5p mediated regulation of the TBK1/IRF3 signaling pathway in vivo and in vitro. Int J Mol Sci 2019;20:3562.
- 30. Martínez-Moreno J, Hernandez JC, Urcuqui-Inchima S. Effect of high doses of vitamin D supplementation on dengue virus replication, Toll-like receptor expression, and cytokine profiles on dendritic cells. Mol Cell Biochem 2020;464:169-180.
- 31. Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr 2003;23:117-145.
- 32. Chel V, Wijnhoven HA, Smit JH, Ooms M, Lips P. Efficacy of different doses and time intervals of oral vitamin D supplementation with or without calcium in elderly nursing home residents. Osteoporos Int 2008;19:663-671.
- 33. Puspitaningtyas H, Sulistyoningrum DC, Witaningrum R, Widodo I, Hardianti MS, Taroeno-Hariadi KW, Kurnianda J, Purwanto I, Hutajulu SH. Vitamin D status in breast cancer cases following chemotherapy: a pre and post observational study in a tertiary hospital in Yogyakarta, Indonesia. PLoS One 2022;17:e0270507.
- 34. Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients 2020;12:2097.
- 35. Jolliffe DA, Vivaldi G, Chambers ES, Cai W, Li W, Faustini SE, Gibbons JM, Pade C, Coussens AK, Richter AG, McKnight Á, Martineau AR. Vitamin D supplementation does not influence SARS-CoV-2 vaccine efficacy or immunogenicity: sub-studies nested within the CORONAVIT randomised controlled trial. Nutrients 2022;14:3821.
- 36. Chillon TS, Demircan K, Heller RA, Hirschbil-Bremer IM, Diegmann J, Bachmann M, Moghaddam A, Schomburg L. Relationship between vitamin D status and antibody response to COVID-19 mRNA vaccination in healthy adults. Biomedicines 2021;9:1714.
- 37. Speakman LL, Michienzi SM, Badowski ME. Vitamins, supplements and COVID-19: a review of currently available evidence. Drugs Context 2021;10:2021-6-2.
- 38. Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, Sudre CH, Nguyen LH, Drew DA, Merino J, Hu C, Selvachandran S, Antonelli M, Murray B, Canas LS, Molteni E, Graham MS, Modat M, Joshi AD, Mangino M, Hammers A, Goodman AL, Chan AT, Wolf J, Steves CJ, Valdes AM, Ourselin S, Spector TD. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: a prospective observational study. Lancet Infect Dis 2021;21:939-949.
- 39. Akbar MR, Wibowo A, Pranata R, Setiabudiawan B. Low serum 25-hydroxyvitamin D (vitamin D) level is associated with susceptibility to COVID-19, severity, and mortality: a systematic review and meta-analysis. Front Nutr 2021;8:660420.
- 40. Petrelli F, Luciani A, Perego G, Dognini G, Colombelli PL, Ghidini A. Therapeutic and prognostic role of vitamin D for COVID-19 infection: a systematic review and meta-analysis of 43 observational studies. J Steroid Biochem Mol Biol 2021;211:105883.
- 41. Brenner H. Vitamin D supplementation to prevent COVID-19 infections and deaths-accumulating evidence from epidemiological and intervention studies calls for immediate action. Nutrients 2021;13:411.
- 42. Rastogi A, Bhansali A, Khare N, Suri V, Yaddanapudi N, Sachdeva N, Puri GD, Malhotra P. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad Med J 2022;98:87-90.
- 43. Kumar P, Kumar M, Bedi O, Gupta M, Kumar S, Jaiswal G, Rahi V, Yedke NG, Bijalwan A, Sharma S, Jamwal S. Role of vitamins and minerals as immunity boosters in COVID-19. Inflammopharmacology 2021;29:1001-1016.
- 44. Thirumdas R, Kothakota A, Pandiselvam R, Bahrami A, Barba FJ. Role of food nutrients and supplementation in fighting against viral infections and boosting immunity: a review. Trends Food Sci Technol 2021;110:66-77.
- 45. Park CS, Shastri N. The role of T cells in obesity-associated inflammation and metabolic disease. Immune Netw 2022;22:e13.
- 46. Dearman RJ, Cumberbatch M, Maxwell G, Basketter DA, Kimber I. Toll-like receptor ligand activation of murine bone marrow-derived dendritic cells. Immunology 2009;126:475-484.
- 47. Zhou Q, Leeman SE, Amar S. Signaling mechanisms involved in altered function of macrophages from diet-induced obese mice affect immune responses. Proc Natl Acad Sci U S A 2009;106:10740-10745.
- 48. Liu F, Guo Z, Dong C. Influences of obesity on the immunogenicity of Hepatitis B vaccine. Hum Vaccin Immunother 2017;13:1014-1017.
- 49. Wypych TP, Marsland BJ, Ubags ND. The impact of diet on immunity and respiratory diseases. Ann Am Thorac Soc 2017;14:S339-S347.
- 50. McNeil MM, Chiang IS, Wheeling JT, Zhang Y. Short-term reactogenicity and gender effect of anthrax vaccine: analysis of a 1967-1972 study and review of the 1955-2005 medical literature. Pharmacoepidemiol Drug Saf 2007;16:259-274.
- 51. Pondo T, Rose CE Jr, Martin SW, Keitel WA, Keyserling HL, Babcock J, Parker S, Jacobson RM, Poland GA, McNeil MM. Evaluation of sex, race, body mass index and pre-vaccination serum progesterone levels and post-vaccination serum anti-anthrax protective immunoglobulin G on injection site adverse events following anthrax vaccine adsorbed (AVA) in the CDC AVA human clinical trial. Vaccine 2014;32:3548-3554.
- 52. Petousis-Harris H, Jackson C, Stewart J, Coster G, Turner N, Goodyear-Smith F, Lennon D. Factors associated with reported pain on injection and reactogenicity to an OMV meningococcal B vaccine in children and adolescents. Hum Vaccin Immunother 2015;11:1875-1880.
- 53. Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 2017;13:851-863.