ABSTRACT
Nutritional support in critically ill patients is an essential aspect of treatment. In particular, the benefits of enteral nutrition (EN) are well recognized, and various guidelines recommend early EN within 48 hours in critically ill patients. However, there is still controversy regarding EN in critically ill patients with septic shock requiring vasopressors. Therefore, this case report aims to provide basic data for the safe and effective nutritional support in septic shock patients who require vasopressors. A 62-year-old male patient was admitted to the intensive care unit with a deep neck infection and mediastinitis that progressed to a septic condition. Mechanical ventilation was initiated after intubation due to progression of respiratory acidosis and deterioration of mental status, and severe hypotension required the initiation of norepinephrine. Due to hemodynamic instability, the patient was kept nil per os. Subsequently, trophic feeding was initiated at the time of norepinephrine dose tapering and was gradually increased to achieve 75% of the energy requirement through EN by the 7th day of enteral feeding initiation. Although there were signs of feeding intolerance during the increasing phase of EN, adjusting the rate of EN resolved the issue. This case report demonstrates the gradual progression and adherence to EN in septic shock patient requiring vasopressors, and the progression observed was relatively consistent with existing studies and guidelines. In the future, further case reports and continuous research will be deemed necessary for safe and effective nutritional support in critically ill patients with septic shock requiring vasopressors.
-
Keywords: Enteral nutrition; Septic shock; Critical illness
INTRODUCTION
Nutritional support in critically ill patients is an essential aspect of treatment. Particularly, enteral nutrition (EN) promotes cholecystokinin secretion, maintains normal gallbladder function, reduces the risk of cholecystitis, supports intestinal mucosa and immune function, and prevents bacterial translocation in the gut are widely recognized [
1]. Therefore, the Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition (SCCM/ASPEN) and the European Society for Clinical Nutrition and Metabolism (ESPEN) recommend early enteral nutrition (EEN) initiating within 48 hours for critically ill patients [
2,
3].
However, the specifics regarding the provision of EN such as optimal timing and amount to patients with septic shock remain unclear. Septic shock involves in hypotension despite adequate fluid resuscitation, therefore requires vasopressor support [
4]. Hemodynamic instability and the use of vasopressors in septic shock patients may contribute to a decrease in splanchnic blood flow and may lead to intestinal ischemia due to EN supply [
5]. Although complications like nonocclusive mesenteric ischemia (NOMI) are rare, they are associated with high mortality rates [
6], so debates regarding timing and amount of EN in patient with septic shock requiring vasopressors are still ongoing, despite recommendations of various existing guidelines about post-resuscitation and hemodynamic stabilization to initiate EEN.
Therefore, in this case report, we report a case of EN in a critically ill patient with septic shock who require vasopressors, and intend to provide basic data about safe and effective nutritional support to septic shock patients. This study was approved by the Institutional Review Board (IRB) of Seoul St. Mary’s Hospital (IRB No. KC23ZISI0828).
CASE
A 62-year-old male patient with diabetes mellitus, hypertension, and dyslipidemia visited a local hospital with a 5-day history of sore throat and dyspnea, but was transferred to Seoul St. Mary’s Hospital due to developed septic condition accompanying deep neck infection and mediastinitis. The patient was admitted to the intensive care unit (ICU) and started mechanical ventilation after intubation caused by progression of respiratory acidosis and decline in mental status. Additionally, severe hypotension necessitated the initiation of norepinephrine. At admission, the patient’s height was 170 cm, weight was 63 kg, and body mass index (BMI) was 21.7 kg/m
2. The Sequential Organ Failure Assessment (SOFA) score was 13, and the Acute Physiology and Chronic Health Evaluation (APACHE) II score was 26. Nutritional screening showed a Nutrition Risk Screening (NRS) 2002 score of 3, and a modified Nutrition Risk in the Critically Ill (mNUTRIC) score of 6. Because of the severity of disease and at a high risk of malnutrition, the patient was referred to the nutrition support team (NST), and nutrition management was applied during ICU stay. The patient’s characteristics presented in
Table 1.
Table 1 Characteristics of patient
Table 1
|
Variables |
Values |
|
Age (yr) |
62 |
|
BMI (kg/m2) |
21.7 |
|
SOFA score |
13 |
|
APACHE II score |
26 |
|
mNUTRIC score |
6 |
|
Intensive care unit (days) |
|
0 |
7 |
14 |
21 |
28 |
|
Biochemical data |
|
|
|
|
|
|
Albumin (g/dL) |
2.0 |
2.7 |
2.5 |
2.8 |
2.9 |
|
CRP (mg/dL) |
51.4 |
8.7 |
4.9 |
8.4 |
2.7 |
|
Hemoglobin (g/dL) |
15.5 |
9.6 |
10.2 |
8.0 |
8.7 |
|
Hematocrit (%) |
47.6 |
27.8 |
30.6 |
23.6 |
25.5 |
|
Platelet (109/L) |
64 |
26 |
41 |
63 |
31 |
|
Blood urea nitrogen (mg/dL) |
59.8 |
37.5 |
36.6 |
73.9 |
70.2 |
|
Creatinine (mg/dL) |
2.8 |
1.6 |
1.0 |
1.7 |
1.3 |
|
Estimated glomerular filtration rate (mL/min/1.73 m2) |
23 |
45 |
83 |
42 |
58 |
|
Nutrition intake |
|
|
|
|
|
|
Total energy intake (kcal/day) |
0 |
1,118 |
1,440 |
600 |
1,600 |
|
Total protein intake (g/day) |
0 |
97 |
86 |
24 |
64 |
ICU #0
The chest computed tomography (CT) scan showed bilateral lower lobe (BLL) pneumonia, and the patient was in septic shock, hypoxia, and deterioration of mental status, so mechanical ventilation was started after intubation. And to raise severe hypotension, norepinephrine was started and increased up to 0.4 mcg/kg/min. To provide appropriate nutritional support, the patient was referred to the NST on the day of admission to the ICU, but due to hemodynamic instability, the patient remained nil per os. The EN supply was decided to proceed after hemodynamic stabilization.
ICU #1
Norepinephrine supply was increased up to a maximum of 0.48 mcg/kg/min, and continuous renal replacement therapy (CRRT) was initiated for hemodynamic instability, septic acute kidney injury, anuria, and metabolic acidosis. An emergency incision & drainage was performed for a deep neck infection. Postoperatively, norepinephrine as well as epinephrine and vasopressin were administered.
ICU #2
Epinephrine and vasopressin were tapered out, and norepinephrine supply was reduced to 0.15 mcg/kg/min. EN was planned to start with a continuous feeding method using a feeding pump. Continuous feeding was initiated at a level of 10 mL (= 10 kcal)/hr via the nasogastric route using a Levin tube and planned to increase by 10 mL/hr per day according to patient’s tolerance. The EN formula supplied was a standard polymer isotonic, high protein, fiber-free product. The patient’s nutritional requirements were calculated at 1,600 kcal/day (25 kcal/kg/day) using a simplistic weight-based equation, and protein set at 95 g/day (1.5 g/kg/day) considering CRRT condition. Insufficient nutrition was supplemented with parenteral nutrition according to the pharmacist’s recommendation, and adjustments in parenteral nutrition were planned as EN increased.
ICU #4–8
Based on tolerance to enteral feedings, the rate gradually increased by 10 mL/hr daily, but there were events in ICU #4 and ICU #6 where regurgitation of EN formula was noted, so enteral feedings were temporarily discontinued and reduced back to the previously tolerated rate. Following this, gradual increments were resumed, and by the 7th day of EN initiation (ICU #8), 75% of the patient’s energy requirements were provided through EN. Norepinephrine supply was maintained at levels between 0.03 to 0.07 mcg/kg/min.
ICU #9–14
Continuous feeding in ICU #9 increased to 60 mL/hr and achieved 90% of energy requirements through EN. PN was discontinued in ICU #9, and norepinephrine supply was tapered out in ICU #10.
ICU #16–17
In ICU #16, the tracheostomy performed and then transitioned to a home ventilator, and in ICU #17, CRRT was discontinued. The protein requirement was modified to 63 g/day (1 g/kg/day) upon discontinuation of CRRT, and the EN formula changed from high protein formula to regular standard formula.
Table 2 shows information of the EN formulas supplied to the patient.
Table 2Composition of the EN formulas
Table 2
|
Composition |
High protein formula |
Regular standard formula |
|
Volume (mL) |
100 |
100 |
|
Energy (kcal) |
100 |
100 |
|
Energy distribution (C:P:F) (%) |
49:24:27 |
58:15:27 |
|
Glucose (g) |
12.3 |
15 |
|
Protein (g) |
6 |
4 |
|
Fat (g) |
3 |
3 |
|
Fiber (g) |
0 |
1.5 |
|
Osmolality (mOsm/kg·H2O) |
300 |
300 |
ICU #21
Due to the improvement in the patient’s clinical condition and the plan for transfer to a general ward, a shift from continuous feeding to intermittent feeding was scheduled. In order to adapt to the increase in feeding rate for transition to intermittent feeding and reduce feeding intolerance, the plan was to start with a rate of 100 mL/hr at 600 mL/3 times/day, and increase the feeding rate and the amount by 100 mL/time daily according to patient’s EN feeding tolerance. Accordingly, during continuous feeding, the supply of EN increased up to a total of 1,440 mL (= 1,440 kcal/d, 90% of the daily energy requirement), but there was a temporary decrease in feeding as it changed to intermittent feeding, but it soon increased as planned. The EN formula was maintained at a regular standard formula.
ICU #28–29
A 100% supply of nutritional requirements was maintained to patient through EN. The patient began taking sips of water to start an oral diet, and ICU based nutrition support was terminated when he was transferred to the general ward later that afternoon.
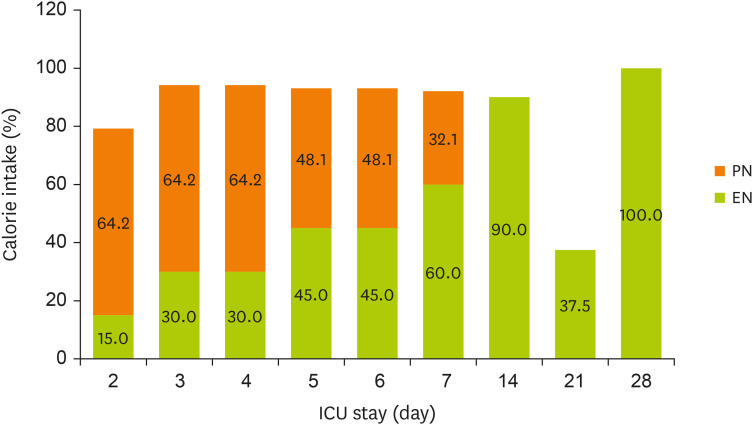
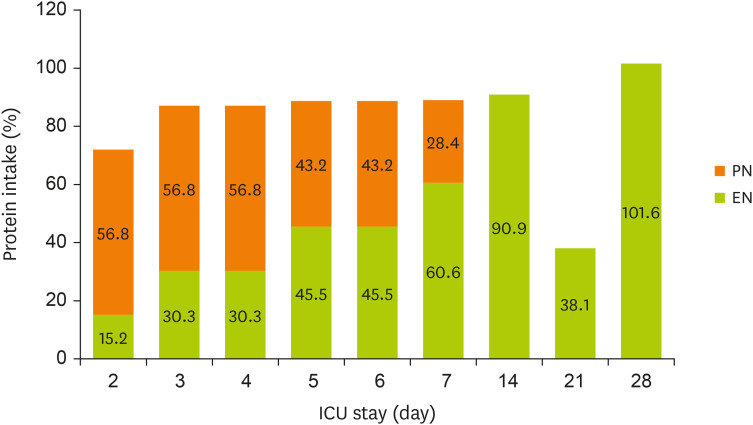
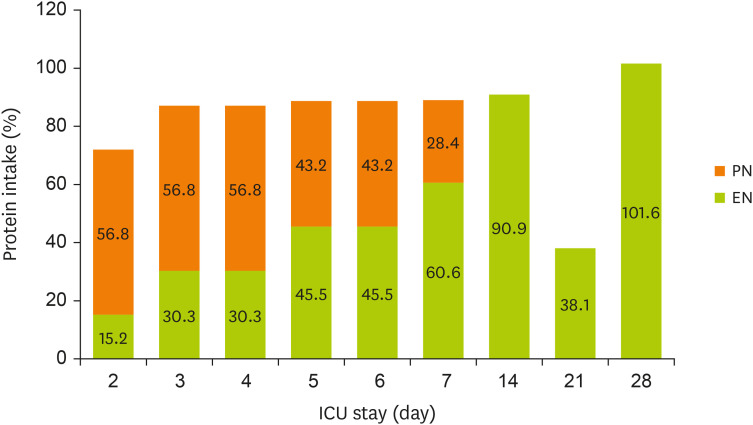
Figures 1 and
2 display the changes in the supplied nutrient amount (%) versus the required amount of nutrition during NST care.
Figure 1
Change in energy intake through EN and PN during the ICU stay.
EN, enteral nutrition; PN, parenteral nutrition; ICU, intensive care unit.
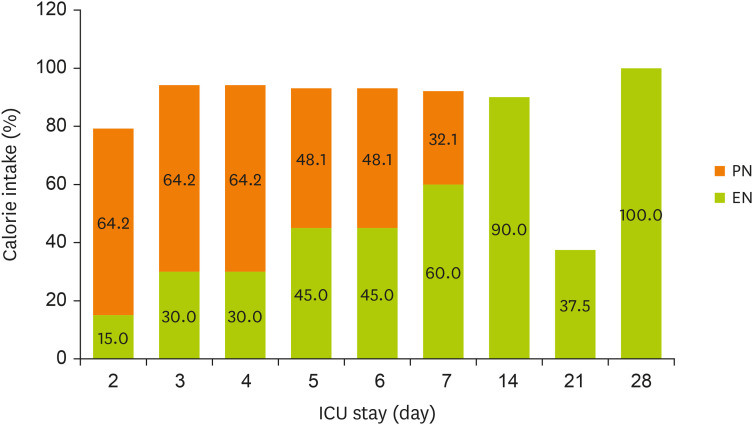
Figure 2
Change in protein intake through EN and PN during the ICU stay.
EN, enteral nutrition; PN, parenteral nutrition; ICU, intensive care unit.
DISCUSSION
The patient in this case report did not immediately start EN at admission to the ICU due to hemodynamic instability. Several guidelines recommend that in hemodynamically unstable situations, enteral feeding should be withheld until fully resuscitated or stabilized [
2,
3,
7]. However, the optimal timing and amount of enteral feeding in critically ill patients with septic shock requiring vasopressors remain unclear, and there are no established cutoff values for safe vasopressor doses.
The 2016 SCCM/ASPEN guidelines suggest enteral feeding within 24–48 hours after a diagnosis of severe sepsis/septic shock as soon as resuscitation is completed and hemodynamically stable [
2]. In 2023, ESPEN did not specify the timing for enteral feeding in septic patients but recommended early enteral feeding after hemodynamic stabilization [
3]. In 2021, the Surviving Sepsis Campaign recommended that EN be initiated within 72 hours for adult patients with sepsis or septic shock [
8].
A study by Ohbe et al. [
9] comparing EEN (< 48 hours) and late EN (> 48 hours) for ventilated adults with shock based on norepinephrine dosage, it suggested that EEN might not be recommended for patients requiring high-dose (≥ 0.3 mcg/kg/min) norepinephrine due to hemodynamic instability [
9]. Merchan et al. [
10] reported that in appropriately fluid-resuscitated septic shock patients receiving less than 0.14 mcg/kg/min of norepinephrine, EEN may be tolerated and safe. Additionally, Mancl and Muzevich’s study [
11] reported that EN was relatively well tolerated in patients receiving IV vasopressor support equivalent to 12.5 mcg/min of norepinephrine or less. The patient in this case report began EEN as suggested by the 2016 SCCM/ASPEN guidelines, with a starting norepinephrine dose of 0.15 mcg/kg/min, which was lower than the norepinephrine equivalent dose found to be relatively tolerable for EN in the studies by Ohbe et al. [
9] and Mancl and Muzevich [
11], and comparable to the norepinephrine equivalent dose in the study by Merchan et al. [
10].
For the initial dose of EN, the 2016 SCCM/ASPEN suggested starting with trophic feeding (defined as 10–20 kcal/h or up to 500 kcal/d) and advancing as tolerated after 24–48 hours to > 80% of target energy goal over the first week [
2], and the 2023 ESPEN recommended starting with a fraction (20%–50%) of full nutrition support and increasing gradually [
3]. The European Society of Intensive Care Medicine recommended starting low-dose EN [
7]. In this case report, we started with trophic feeding at a level of 10 mL (= 10 kcal)/hr following the 2016 SCCM/ASPEN guidelines. Continuous feeding was planned to reduce gastrointestinal side effects. By the 7th day of initiating EN, 75% of the energy requirement was provided through EN, and 90% of requirement was reached by the 8th day.
Intolerance to EN in hemodynamically unstable patients requiring vasopressors is relatively common, with Merchan et al. [
10] reporting “intolerance to EN” in 38% of patients and Flordelis Lasierra et al. [
12] reporting “EN-related complication” in 77% of patients. The patient in this case report experienced regurgitation, which was resolved by temporarily discontinuing EN or reducing the feeding rate. As suggested by the study of Piton et al. [
6], in critically ill patients with septic shock requiring vasopressors, providing full EN based on target energy with EEN might be less safe concerning complications like NOMI compared to trophic feeding. However, gradually increasing from low-dose EEN, as observed in this case, might allow relatively safe and tolerable provision of EN.
This case report showed the gradual progression and compliance of EEN in a critically ill patient with septic shock requiring vasopressors, and the progression observed was relatively in line with existing studies and guidelines. However, the medical condition and severity of septic shock patients vary, and it is difficult to clearly define the hemodynamic stability of critically ill patients. Therefore, for safe and effective EN, further case reports and studies on septic shock patients requiring vasopressors will be needed.
NOTES
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Kim HY.
Data curation: Kim HY.
Formal analysis: Kim HY.
Investigation: Kim HY.
Methodology: Kim HY.
Project administration: Kim HY.
Supervision: Kim HY.
Visualization: Kim HY.
Writing - original draft: Kim HY.
Writing - review & editing: Noh MY, Lee J.
REFERENCES
- 1. Mueller CM, Lord LM, Marian M, McClave SA, Miller SJ. Overview of enteral nutrition. In Doley J, Phillips W, eds, ddThe ASPEN adult nutrition support core curriculum. 3rd ed. Silver Spring (MD): American Society for Parenteral and Enteral Nutrition; 2017, pp 213-223.
- 2. McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, Gervasio JM, Sacks GS, Roberts PR, Compher C. Society of Critical Care Medicine. American Society for Parenteral and Enteral Nutrition. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016;40:159-211.
- 3. Singer P, Blaser AR, Berger MM, Calder PC, Casaer M, Hiesmayr M, Mayer K, Montejo-Gonzalez JC, Pichard C, Preiser JC, Szczeklik W, van Zanten AR, Bischoff SC. ESPEN practical and partially revised guideline: clinical nutrition in the intensive care unit. Clin Nutr 2023;42:1671-1689.
- 4. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801-810.
- 5. Allen JM. Vasoactive substances and their effects on nutrition in the critically ill patient. Nutr Clin Pract 2012;27:335-339.
- 6. Piton G, Le Gouge A, Boisramé-Helms J, Anguel N, Argaud L, Asfar P, Botoc V, Bretagnol A, Brisard L, Bui HN, Canet E, Chatelier D, Chauvelot L, Darmon M, Das V, Devaquet J, Djibré M, Ganster F, Garrouste-Orgeas M, Gaudry S, Gontier O, Groyer S, Guidet B, Herbrecht JE, Hourmant Y, Lacherade JC, Letocart P, Martino F, Maxime V, Mercier E, Mira JP, Nseir S, Quenot JP, Richecoeur J, Rigaud JP, Roux D, Schnell D, Schwebel C, Silva D, Sirodot M, Souweine B, Thieulot-Rolin N, Tinturier F, Tirot P, Thévenin D, Thiéry G, Lascarrou JB, Reignier J. Clinical Research in Intensive Care and Sepsis (CRICS) group. Factors associated with acute mesenteric ischemia among critically ill ventilated patients with shock: a post hoc analysis of the NUTRIREA2 trial. Intensive Care Med 2022;48:458-466.
- 7. Reintam Blaser A, Starkopf J, Alhazzani W, Berger MM, Casaer MP, Deane AM, Fruhwald S, Hiesmayr M, Ichai C, Jakob SM, Loudet CI, Malbrain ML, Montejo González JC, Paugam-Burtz C, Poeze M, Preiser JC, Singer P, van Zanten AR, De Waele J, Wendon J, Wernerman J, Whitehouse T, Wilmer A, Oudemans-van Straaten HM. ESICM Working Group on Gastrointestinal Function. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med 2017;43:380-398.
- 8. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Møller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021;47:1181-1247.
- 9. Ohbe H, Jo T, Matsui H, Fushimi K, Yasunaga H. Differences in effect of early enteral nutrition on mortality among ventilated adults with shock requiring low-, medium-, and high-dose noradrenaline: a propensity-matched analysis. Clin Nutr 2020;39:460-467.
- 10. Merchan C, Altshuler D, Aberle C, Papadopoulos J, Schwartz D. Tolerability of enteral nutrition in mechanically ventilated patients with septic shock who require vasopressors. J Intensive Care Med 2017;32:540-546.
- 11. Mancl EE, Muzevich KM. Tolerability and safety of enteral nutrition in critically ill patients receiving intravenous vasopressor therapy. JPEN J Parenter Enteral Nutr 2013;37:641-651.
- 12. Flordelís Lasierra JL, Montejo González JC, López Delgado JC, Zárate Chug P, Martínez Lozano-Aranaga F, Lorencio Cárdenas C, Bordejé Laguna ML, Maichle S, Terceros Almanza LJ, Trasmonte Martínez MV, Mateu Campos L, Servià Goixart L, Vaquerizo Alonso C, Vila García B. the NUTRIVAD Study Group. Enteral nutrition in critically ill patients under vasoactive drug therapy: the NUTRIVAD study. JPEN J Parenter Enteral Nutr 2022;46:1420-1430.