ABSTRACT
Calcium plays a major role in apoptosis, cell proliferation, and various cellular mechanisms. It is also essential for the function of the pancreas. However, the association between calcium intake and pancreatic cancer is not clear. This study aims to clarify the links between calcium intake and pancreatic cancer risk using a systematic review and meta-analysis of observational studies. PubMed, Web of Science, Scopus, and Google Scholar were searched for eligible articles published through 31 August 2023. Case-control and cohort studies reporting the association between dietary and/or supplemental calcium intake and risk of pancreatic cancer using relative risk (RR), hazard ratio (HR), or odds ratio (OR) with 95% confidence interval (CI) were included. Meta-analysis using a random effect model was used to estimate the significance of the association. Eight studies were included. An inverse association between total calcium intake (dietary and supplement) and pancreatic cancer risk (RR, 0.83; 95% CI, 0.72–0.97; I2 = 0%) was observed. However, the association between dietary calcium intake alone and pancreatic cancer risk did not reach a statistically significant level (RR, 0.91; 95% CI, 0.78–1.06; I2 = 48%). Higher total calcium intake may reduce the risk of pancreatic cancer but the difference between sources of calcium (dietary vs. supplementation) requires further investigation. Also, due to the heterogeneity between the articles, the results of this study should be interpreted with caution.
-
Trial Registration
-
Keywords: Calcium; Dietary; Supplementary calcium; Pancreatic cancer
INTRODUCTION
In most developed countries, pancreatic cancer is among the top five leading causes of cancer-related death in both men and women [
1,
2,
3]. Smoking, overweight and obesity, alcohol consumption, diabetes and poor dietary intake (high intake of animal products and low intake of fruit and vegetables and some micronutrients) are modifiable risk factors for pancreatic cancer [
4,
5]. Calcium is an important micronutrient, essential for bone health, muscle and nerve function, and regulation of hormones [
6]. Calcium intake has been associated with different conditions such as cardiovascular diseases, strokes, and osteoporosis [
7,
8,
9]. The association between calcium intake and different types of cancer is inconsistent. A meta-analysis of 15 cohort studies, suggested a potential protective relationship between calcium intake and colorectal cancer [
10]. Similarly, an inverse dose-response relationship between calcium intake and breast cancer risk has been reported [
11]. However, a meta-analysis found that dairy calcium intake, but not supplemental or non-dairy calcium is associated with a higher risk of prostate cancer [
12], while another meta-analysis suggested that total calcium intake (dietary and supplemental) is associated with a higher risk of prostate cancer [
13].
There is also growing evidence from experimental and human studies suggesting a link between calcium and pancreatic cancer [
14,
15]. Pancreas sulfonylurea receptors control intracellular calcium changes, which play a role in the modulation of adiposity and body fat [
16]. High intake of calcium may accelerate lipolysis, decrease lipogenesis, and increase fecal fat excretion [
16,
17], potentially reducing the risk of pancreatic cancer [
18]. However, epidemiological studies investigating the association between dietary calcium and pancreatic cancer risk resulted in conflicting findings with some studies reporting an increased risk of pancreatic cancer [
15] while others suggested a protective relationship [
18,
19,
20,
21], or no association [
14,
22,
23]. Therefore, this systematic review and meta-analysis aims to clarify the associations between calcium (dietary or total) intake and the risk of pancreatic cancer.
MATERIALS AND METHODS
The Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines were followed during the preparation and presentation of this meta-analysis [
24]. The protocol for this meta-analysis was registered in PROSPERO (
CRD42022331647).
Observational studies investigated the relationship between calcium intake and pancreatic cancer risk were searched in PubMed, Scopus, and ISI Web of Science databases through 31st August 2023. Google Scholar and the reference lists of the included papers and recent review articles on this topic were also searched. Following Population, Intervention, Comparison, and Outcome (PICO) framework (
Table 1) a combination of the following search keywords or phrases were used to find relevant literature: ((("ca"[Title/Abstract] OR "calcium*"[Title/Abstract] OR "dairy*"[Title/Abstract] OR "milk*"[Title/Abstract] OR "cheese*"[Title/Abstract] OR "yogurt*"[Title/Abstract] OR "miner*"[Title/Abstract]) AND ("diet*"[Title/Abstract] OR "food*"[Title/Abstract] OR "nutr*"[Title/Abstract] OR "intak*"[Title/Abstract] OR "healthy study"[Title/Abstract])) AND ("cancer*"[Title/Abstract] OR "tumor*"[Title/Abstract] OR "neopla*"[Title/Abstract] OR "malignan*"[Title/Abstract] OR "carcin*"[Title/Abstract])) AND ("pancr*"[Title/Abstract] OR "duct gland*"[Title/Abstract] OR "island of Langerhans"[Title/Abstract] OR "insulin secretion cells"[Title/Abstract]) (
Supplementary Table 1).
Table 1The PICO criteria used for the present systematic review and meta-analysis
Table 1
|
PICO criteria |
Description |
|
Patients |
Healthy adult subjects or adult patient with pancreatic cancer |
|
Exposure |
“Dietary calcium intake” OR “Supplementary calcium intake” |
|
Comparison |
The highest calcium intake versus the lowest calcium intake |
|
Outcome |
Pancreatic cancer |
Selection criteria
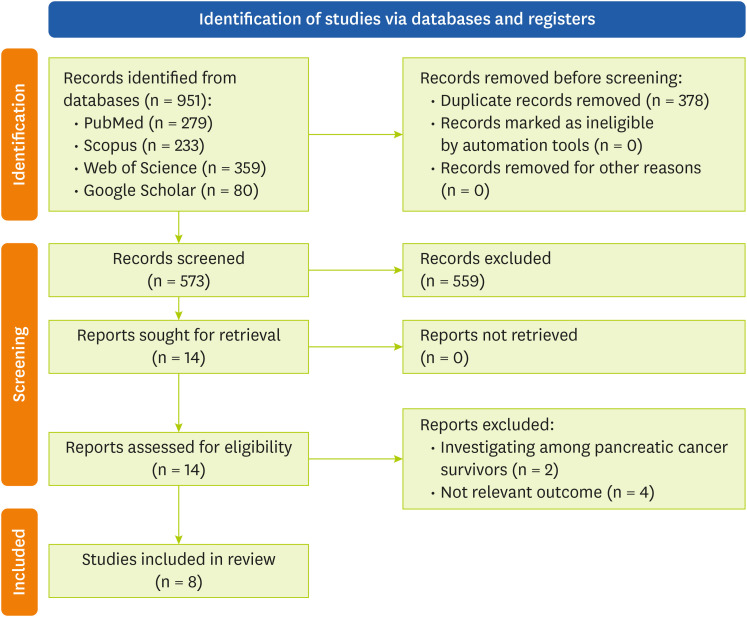
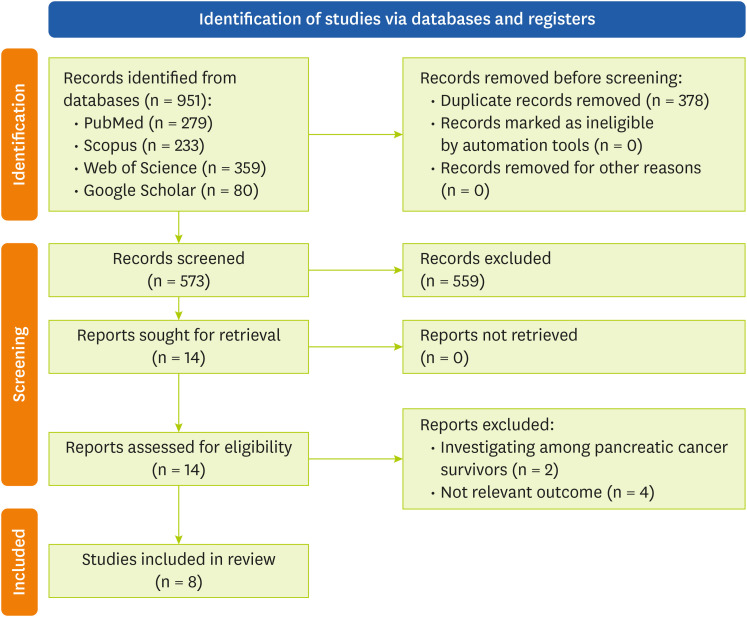
Studies with a case-control or cohort design that report the association between calcium intake and pancreatic cancer risk as odds ratio (OR), risk ratios (RRs) or hazard ratios (HRs) with corresponding 95% confidence intervals (CIs), with accessible full-text published in English were included. Review and meta-analysis articles, laboratory and experimental studies, and studies with insufficient data were excluded. The screening process started by reviewing the titles and abstracts of the searched articles. Then, the full text of related studies was reviewed. The review process and study selection are illustrated in
Figure 1.
Figure 1Flow chart of literature search process.
Data extraction and quality assessment
Data extraction from eligible articles was performed independently by 2 reviewers (MM, FA). Information on first author’s name, year of publication, location, design, duration (for cohort studies), number of controls/cases (for case-control studies), number of participants and cases (for cohort studies), methods of dietary assessment (food frequency questionnaire, dietary history questionnaire, etc.), type of calcium intake (dietary, dietary + supplementary, and supplementary), OR, HR or RR with 95% CI and adjusted variables were extracted. The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the studies [
25]. Studies were evaluated by NOS based on selection, comparison of groups and determination of results, with a quality score between 0 and 9. NOS scores < 4, 4 to 6, and > 6 were considered low, medium, and high quality, respectively. Quality assessment was completed independently by 2 reviewers (FA, AP) and any disagreements were resolved by involving a third investigator (EH).
All statistical analyses were conducted using RStudio software, version 1.3.1073 [
26]. The incidence of lung cancer was summarized as RRs. A DerSimonian and Laird random-effect meta-analysis was used to compare the risk of pancreatic cancer between the highest and lowest levels of calcium intake [
27]. Heterogeneity among studies was tested using χ
2 and I
2 test statistics according to the Cochrane classifications (I
2 < 25%, 25%–75%, and > 75% indicating low, medium, and high heterogeneity, respectively) [
28]. To explore heterogeneity sources, the priori subgroup analyses of gender and study design were conducted. Also, a sensitivity analysis based on the leave-one-out method [
29] was performed to explore the influence of individual studies on the overall meta-analysis effect and heterogeneity. Significant changes in overall meta-analysis effects or heterogeneity influenced by excluding one study at a time suggested the sensitivity of the overall meta-analysis to the excluded study. Publication bias was assessed using funnel plot and Egger’s test, with p < 0.05 representing a significant publication bias. Also to adjust for funnel plot asymmetry, the Duval and Tweedie trim-and-fill analysis was performed [
30].
RESULTS
Literature research and studies characteristics
Eight studies met the eligibility criteria and were included in this systematic review and meta-analysis [
14,
15,
18,
19,
20,
21,
22,
23]. The characteristics of the included studies are shown in
Table 2. Six studies were conducted in the United States [
14,
15,
18,
19,
20,
21], one in Italy [
22], and one in Greece [
23]. Three studies had a cohort design [
19,
20,
21] and five were case-control studies [
14,
15,
18,
22,
23]. The total number of participants in the cohort studies was 854,381, of which 2,702 eventually developed pancreatic cancer, with a follow-up period of 7 to 12.2 years. The total number of participants in the case-control studies was 1,325 (for cases) and 3,179 (for controls). All studies included both genders as participants, except for one case-control study that included only men [
18]. All 8 studies examined the relationship between pancreatic cancer risk and dietary calcium, but only 4 assessed the relationship with total (dietary and supplemental) calcium (2 were cohort studies) [
14,
15,
19,
20]. In 6 studies, food frequency questionnaire was used to evaluate diet. Another 2 studies collected dietary assessments from dietary segments of the Health Habits and History Questionnaire [
18] and the 124-item dietary history questionnaire [
19]. Additionally, 6 studies collected dietary data 1 year before cancer diagnosis [
14,
15,
19,
20,
21,
23], one study collected data 2 years before diagnosis [
22], and one study collected data three years before diagnosis [
18]. Based on NOS evaluation, 5 studies were deemed as high quality (NOS > 7) and three as moderate quality (7 > NOS > 4). Details of NOS scoring for each study are shown in
Supplementary Tables 2 and
3.
Table 2Main characteristics of included studies
Table 2
|
Study (author, year) |
Location |
Design |
Duration |
Participants/cases (cohort) |
Age (range) |
Controls/cases |
Dietary assessment |
Type of calcium |
RR/OR/HR (95% CI) of highest vs. lowest intake of dietary calcium |
Adjusted variables |
NOS |
|
Farrow and Davis, 1990 [18] |
USA |
Case-control |
NA |
- |
30–74 |
186/137 |
Dietary segment of the Health Habits and History Questionnaire |
- Dietary calcium |
Dietary calcium: Men, 0.5 (0.2–1.00) |
Age, smoking, education, calorie-adjusted protein intake |
5/9 |
|
Kalapothaki et al., 1993 [23] |
Greece |
Case-control |
NA |
- |
NA |
181/181 |
110 Food item FFQ |
- Dietary calcium |
Dietary calcium: Overall, 1.02 (0.85–1.23) |
Age, gender, cigarette smoking, diabetes mellitus, energy intake |
5/9 |
|
Park et al., 2009 [20] |
USA |
Cohort |
7 years |
492,810/1,101 |
50–71 |
- |
124 Food item FFQ |
- Dietary calcium |
Dietary calcium: Men, 0.82 (0.64–1.06); Women, 0.85 (0.60–1.20) |
Race, education, BMI, physical activity, smoking status, diabetes, hypertension |
6/9 |
|
- Total calcium |
Total calcium: Men, 0.87 (0.68–1.11); Women, 0.88 (0.63–1.24) |
|
- Supplemental calcium |
Supplemental calcium: Men, 1.17 (0.77–1.77); Women, 0.79 (0.57–1.11) |
|
Bravi et al., 2011 [22] |
Italy |
Case-control |
NA |
- |
34–80 |
652/326 |
78 Food item FFQ |
- Dietary calcium |
Dietary calcium: Overall, 1.51 (0.90–2.52) |
Education, tobacco smoking, history of diabetes, BMI, total energy intake, |
7/9 |
|
Zablotska et al., 2011 [15] |
USA |
Case-control |
NA |
- |
50–69 |
1,701/532 |
131 Food item FFQ |
- Dietary calcium |
Dietary calcium: Men, 2.8 (1.2–6.4); Women, 0.70 (0.27–1.8) |
Energy intake, age, BMI, education, smoking, history of diabetes, physical activity, alcohol consumption |
7/9 |
|
- Total calcium |
Total calcium: Men, 1.2 (0.68–2.1); Women, 0.81 (0.45–1.4) |
|
Gordon-Dseagu et al., 2017 [21] |
USA |
Cohort |
10 years |
303,094/1,322 |
50–71 |
- |
37 Food item FFQ |
- Dietary calcium |
Dietary calcium: Overall, 0.87 (0.76–0.99) |
Sex and energy intake, smoking, BMI, self-reported diabetes |
7/9 |
|
Fan et al., 2021 [14] |
USA |
Case-control |
NA |
- |
20–64 |
459/150 |
131-item Willett FFQ |
- Dietary calcium |
Dietary calcium: Overall, 0.72 (0.38–1.37) |
Age, sex, race, education cigarette smoking alcohol consumption total energy |
9/9 |
|
- Total calcium |
Total calcium: Overall, 0.69 (0.37–1.28) |
|
Hoyt et al., 2021 [19] |
USA |
Cohort |
12.2 years |
58,477/279 |
55–74 |
- |
124-item DHQ |
- Dietary calcium |
Dietary calcium: Overall, 0.73 (0.49–1.07) |
Age, sex, race, BMI, diabetes status, cigarette, energy intake |
7/9 |
|
- Total calcium |
Total calcium: Overall, 0.67 (0.47–0.96) |
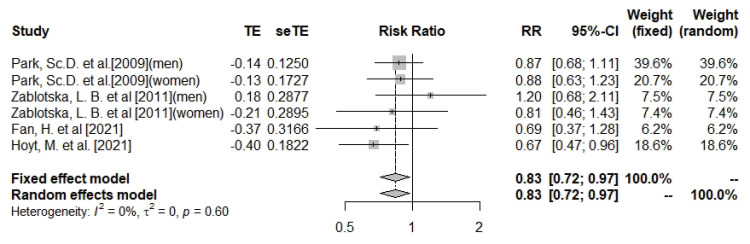
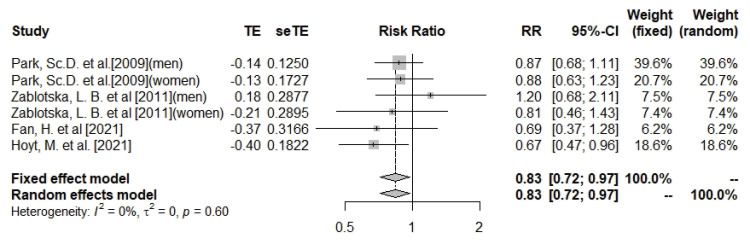
Total calcium intake and pancreatic cancer
This meta-analysis found that total calcium (dietary and supplemental) intake was significantly associated with a 17% lower risk of pancreatic cancer (n = 6; RR, 0.83; 95% CI, 0.72–0.97; I
2 = 0%; heterogeneity p = 0.6) (
Figure 2). The subgroup of cohort studies also showed a similar relationship between total calcium intake and pancreatic cancer (n = 3; RR, 0.82; 95% CI, 0.69–0.98; I
2 = 0%; heterogeneity p = 0.44), but the association did not reach a significant level in case-control studies (
Supplementary Figures 1 and
2). Subgroup analysis by gender did not result in statistically significant associations (
Supplementary Figures 3 and
4). There was no evidence of asymmetry among studies based on the funnel plot and Eggers’ test (Egger’s test p value for total calcium = 0.99;
Supplementary Figure 5). Due to the absence of heterogeneity, sensitivity analyses to investigate sources of heterogeneity were not performed.
Figure 2
Forest plot of total calcium and risk of pancreatic cancer.
TE, the calculated within-group effect size; seTE, the standard error of the within-group effect size; RR, risk ratio; CI, confidence interval.
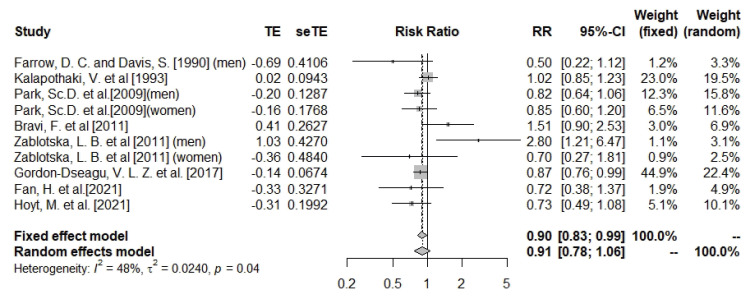
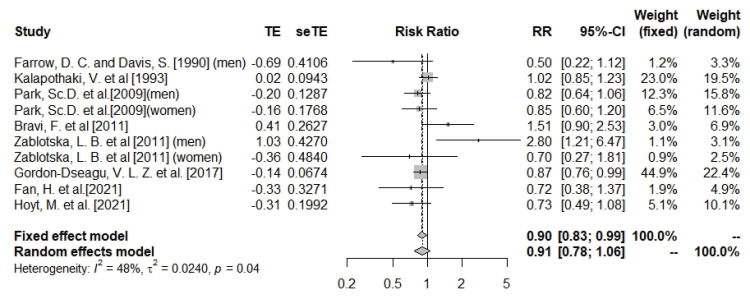
Dietary calcium intake and pancreatic cancer
The meta-analysis results suggested no significant association between dietary calcium intake and pancreatic cancer risk (n = 10; RR, 0.91; 95% CI, 0.78–1.06; I
2 = 48%; heterogeneity p = 0.04) (
Figure 3). The association was significant in the subgroup analysis of cohort studies (n = 4; RR, 0.85; 95% CI, 0.76–0.94; I
2 = 0%; heterogeneity p = 0.85), but did not reach a statistically significant level in the subgroup of case-control studies (
Supplementary Figures 6 and
7). Subgroup analysis by gender did not result in statistically significant associations (
Supplementary Figures 8 and
9). Meta analysis was sensitive to Zablotska et al.’s study [
15]. Removal of this study reduced heterogeneity and changed the overall direction of results (RR ranged between 0.81 and 0.97, I
2 = 23%) (
Supplementary Figure 10). Although there was evidence of asymmetry based on the funnel plot (
Supplementary Figure 11), Egger’s test showed no evidence of publication bias (Egger’s p-test value for dietary calcium = 0.82).
Figure 3
Forest plot of dietary calcium and risk of pancreatic cancer.
TE, the calculated within-group effect size; seTE, the standard error of the within-group effect size; RR, risk ratio; CI, confidence interval.
DISCUSSION
This meta-analysis investigated the association between calcium (dietary and/or supplement) intake and the risk of pancreatic cancer. Overall, the results suggested that higher total calcium (dietary and supplement) intake is associated with a lower risk of pancreatic cancer, but a similar association was not observed for dietary calcium intake alone. Subgroup analysis based on study design suggested a negative relationship between dietary and total calcium intake with pancreatic cancer risk in cohort studies. However, the associations were not different based on subgroup analyses of gender. Similar to the findings of the present study, no significant association between dietary calcium and pancreatic cancer risk was reported in a previous meta-analysis of 14 cohort studies conducted in 2014 [
31].
According to Zablotska et al. [
15], calcium intake from food has been associated with a 2.8-fold increased risk of pancreatic cancer among men. However, other studies have not found a significant relationship between calcium intake (whether from dietary sources or with supplements), and pancreatic cancer risk [
14,
22]. Also, a cohort study consistent with the findings of the present study demonstrated that total calcium consumption can actually reduce the risk of pancreatic cancer [
19]. All the studies discussed in this article are observational, examining the relationship between dietary and supplemental calcium intake and the risk of pancreatic cancer. These studies include both prospective and retrospective designs. In prospective studies, participants are initially cancer-free, and cancer cases develop during the study period, providing a stronger causal relationship compared to retrospective studies
Although the exact protective mechanisms of calcium against pancreatic cancer are unclear, the ability of calcium to regulate cell apoptosis may explain the findings of this study [
32]. Apoptosis is triggered by the release of intracellular calcium into the cytoplasm. Calcium accumulates within the mitochondria and opens the mitochondrial permeability transition pore [
32]. This can result in mitochondrial dysfunction, loss of membrane potential, and the release of pro-apoptotic proteins like cytochrome C, leading to cell death [
32]. Calcium alone and through interactions with vitamin D may be responsible for its anticarcinogenic potential and for initiating apoptosis [
33,
34]. Cellular calcium is responsible for about 2/3 of the T cell gene expression activation or suppression [
35,
36]. Once they proliferate, mature, and acquire effector status, T cells exit the lymph nodes and circulate to locate cancer cells and accumulate within cancerous tissues [
35]. This process subsequently triggers the recruitment of other chemokines, enhancing the efficiency of the immune response against cancer [
35]. There is also an interaction between total calcium intake and total fat intake concerning pancreatic cancer risk [
19]. In individuals with a high total fat intake, increased calcium intake is associated with a lower pancreatic cancer risk [
19]. This could be due to an increase in fecal fat excretion due to calcium soap formation in the gut [
10,
37]. This, in turn, reduces the adverse effects of excess fat on the body including insulin resistance, dysregulated autophagy, and persistent inflammation [
38,
39].
This meta-analysis also reported differences in the association between dietary and total calcium concerning pancreatic cancer risk. These findings are difficult to justify due to various factors influencing the bioavailability of calcium [
40]. For example, calcium bioavailability can be impacted by the presence of other nutrients, components, or contaminants within diet or supplement, and an individual’s vitamin D and calcium status [
41]. Also, this study observed a significant inverse relationship between total calcium intake and the risk of pancreatic cancer, this association may vary across different stages of the cancer. Therefore, further studies are needed to examine these issues between calcium intake and the various stages of pancreatic cancer.
The current study had some strengths. It investigated the association between dietary and total calcium intake and pancreatic cancer risk by pooling the results from eight case-control and cohort studies and increasing the statistical power of the findings. However, the study also had some limitations. To improve comparability, different effect sizes (OR, HR) were considered equivalent to RR. However, these effect sizes vary in statistical nature and interpretation. Observational studies are also generally limited by the quality of the data collected, especially when using self-reported questionnaires, with the potential for selection bias [
42]. Also, the power of meta-analysis tests was limited due to the small number of included studies (< 10) [
43]. The studies collected data over different time periods (e.g., one study collected data 1 year before cancer diagnosis, while another collected data 3 years before diagnosis). These variations can lead to differing overall results and introduce various biases, including recall bias, which should be considered another limitation of our study. Nevertheless, the findings of this study provide important information for future interventions and guidelines to reduce the risk of pancreatic cancer.
CONCLUSION
The results of the current systematic review and meta-analysis suggest that high total calcium intake is associated with a lower risk of pancreatic cancer risk. The results indicated a 17% lower risk of pancreatic cancer with high total calcium intake. Furthermore, given the observed heterogeneity amongst the articles under consideration, it is prudent to approach the interpretation of this study’s findings with a degree of caution.
NOTES
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Mohammadzadeh M.
Data curation: Mohammadzadeh M, Abdi F, Paydareh A.
Formal analysis: Bahrami A.
Validation: Mohammadzadeh M.
Writing - original draft: Mohammadzadeh M, Abdi F, Hejazi E.
Writing - review & editing: Bahrami A, Mohammadzadeh M, Khalesi S, Hejazi E.
SUPPLEMENTARY MATERIALS
Supplementary Table 2
Quality assessment using New Castle-Ottawa Scale for cohort studies
cnr-13-284-s002.xls
Supplementary Table 3
Quality assessment using New Castle-Ottawa Scale for case-control studies
cnr-13-284-s003.xls
Supplementary Figure 1
Forest plot of total calcium (dietary + supplemental) and risk of pancreatic cancer in cohort studies.
cnr-13-284-s004.ppt
Supplementary Figure 2
Forest plot of total calcium (dietary + supplemental) and risk of pancreatic cancer in case-control studies.
cnr-13-284-s005.ppt
Supplementary Figure 3
Forest plot of total calcium (dietary + supplemental) and risk of pancreatic cancer among men.
cnr-13-284-s006.ppt
Supplementary Figure 4
Forest plot of total calcium (dietary + supplemental) and risk of pancreatic cancer among women.
cnr-13-284-s007.ppt
Supplementary Figure 5
Begg’s funnel plot (with pseudo 95% confidence interval) depicting log RR against their corresponding SE for assessing the presence of publication bias in studies that investigated the association between dietary total calcium (dietary + supplemental) and risk of pancreatic cancer.
cnr-13-284-s008.ppt
Supplementary Figure 6
Forest plot of dietary calcium and risk of pancreatic cancer among cohort studies.
cnr-13-284-s009.ppt
Supplementary Figure 7
Forest plot of dietary calcium and risk of pancreatic cancer among case-control studies.
cnr-13-284-s010.ppt
Supplementary Figure 8
Forest plot of dietary calcium and risk of pancreatic cancer among men.
cnr-13-284-s011.ppt
Supplementary Figure 9
Forest plot of dietary calcium and risk of pancreatic cancer among women.
cnr-13-284-s012.ppt
Supplementary Figure 10
Sensitivity analysis based on the Leave-One-Out-method sort by I2 for dietary calcium and risk of pancreatic cancer.
cnr-13-284-s013.ppt
Supplementary Figure 11
Begg’s funnel plot (with pseudo 95% confidence interval) depicting log RR against their corresponding SE for assessing the presence of publication bias in studies that investigated the association between dietary calcium and risk of pancreatic cancer.
cnr-13-284-s014.ppt
REFERENCES
- 1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-2921.
- 2. Bradford N, Pitt E, Alexander K. Research priorities of Australian cancer nurses: a national consensus survey. Collegian 2022;29:566-573.
- 3. Canadian Cancer Statistics Advisory Committee. Canadian cancer statistics 2022. 2022. cited 2023 November 18. Available from https://cancer.ca/en/research/cancer-statistics
- 4. Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694-9705.
- 5. Iodice S, Gandini S, Maisonneuve P, Lowenfels AB. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg 2008;393:535-545.
- 6. Power ML, Heaney RP, Kalkwarf HJ, Pitkin RM, Repke JT, et al. The role of calcium in health and disease. Am J Obstet Gynecol 1999;181:1560-1569.
- 7. Asemi Z, Saneei P, Sabihi SS, Feizi A, Esmaillzadeh A. Total, dietary, and supplemental calcium intake and mortality from all-causes, cardiovascular disease, and cancer: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis 2015;25:623-634.
- 8. Larsson SC, Orsini N, Wolk A. Dietary calcium intake and risk of stroke: a dose-response meta-analysis. Am J Clin Nutr 2013;97:951-957.
- 9. Peterlik M, Kállay E, Cross HS. Calcium nutrition and extracellular calcium sensing: relevance for the pathogenesis of osteoporosis, cancer and cardiovascular diseases. Nutrients 2013;5:302-327.
- 10. Keum N, Aune D, Greenwood DC, Ju W, Giovannucci EL. Calcium intake and colorectal cancer risk: dose-response meta-analysis of prospective observational studies. Int J Cancer 2014;135:1940-1948.
- 11. Hidayat K, Chen GC, Zhang R, Du X, Zou SY, et al. Calcium intake and breast cancer risk: meta-analysis of prospective cohort studies. Br J Nutr 2016;116:158-166.
- 12. Aune D, Navarro Rosenblatt DA, Chan DS, Vieira AR, Vieira R, et al. Dairy products, calcium, and prostate cancer risk: a systematic review and meta-analysis of cohort studies. Am J Clin Nutr 2015;101:87-117.
- 13. Rahmati S, Azami M, Delpisheh A, Hafezi Ahmadi MR, Sayehmiri K. Total calcium (dietary and supplementary) intake and prostate cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev 2018;19:1449-1456.
- 14. Fan H, Yu Y, Nan H, Hoyt M, Reger MK, et al. Associations between intake of calcium, magnesium and phosphorus and risk of pancreatic cancer: a population-based, case-control study in Minnesota. Br J Nutr 2021;126:1549-1557.
- 15. Zablotska LB, Gong Z, Wang F, Holly EA, Bracci PM. Vitamin D, calcium, and retinol intake, and pancreatic cancer in a population-based case-control study in the San Francisco Bay area. Cancer Causes Control 2011;22:91-100.
- 16. Zemel MB. Mechanisms of dairy modulation of adiposity. J Nutr 2003;133:252S-256S.
- 17. Soerensen KV, Thorning TK, Astrup A, Kristensen M, Lorenzen JK. Effect of dairy calcium from cheese and milk on fecal fat excretion, blood lipids, and appetite in young men. Am J Clin Nutr 2014;99:984-991.
- 18. Farrow DC, Davis S. Diet and the risk of pancreatic cancer in men. Am J Epidemiol 1990;132:423-431.
- 19. Hoyt M, Song Y, Gao S, O’Palka J, Zhang J. Intake of calcium, magnesium, and phosphorus and risk of pancreatic cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. J Am Nutr Assoc 2022;41:747-757.
- 20. Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med 2009;169:391-401.
- 21. Gordon-Dseagu VL, Thompson FE, Subar AF, Ruder EH, Thiébaut AC, et al. A cohort study of adolescent and midlife diet and pancreatic cancer risk in the NIH-AARP diet and health study. Am J Epidemiol 2017;186:305-317.
- 22. Bravi F, Polesel J, Bosetti C, Talamini R, Negri E, et al. Dietary intake of selected micronutrients and the risk of pancreatic cancer: an Italian case-control study. Ann Oncol 2011;22:202-206.
- 23. Kalapothaki V, Tzonou A, Hsieh CC, Karakatsani A, Trichopoulou A, et al. Nutrient intake and cancer of the pancreas: a case-control study in Athens, Greece. Cancer Causes Control 1993;4:383-389.
- 24. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-2012.
- 25. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2014. cited 2023 November 18. Available from https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 26. RStudio Team. RStudio: integrated development environment for R. 2020. cited 2023 November 18. Available from http://www.rstudio.com/
- 27. Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med 2010;29:1282-1297.
- 28. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-560.
- 29. Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods 2010;1:112-125.
- 30. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-463.
- 31. Genkinger JM, Wang M, Li R, Albanes D, Anderson KE, et al. Dairy products and pancreatic cancer risk: a pooled analysis of 14 cohort studies. Ann Oncol 2014;25:1106-1115.
- 32. Zhivotovsky B, Orrenius S. Calcium and cell death mechanisms: a perspective from the cell death community. Cell Calcium 2011;50:211-221.
- 33. Hajnóczky G, Davies E, Madesh M. Calcium signaling and apoptosis. Biochem Biophys Res Commun 2003;304:445-454.
- 34. Mathiasen IS, Sergeev IN, Bastholm L, Elling F, Norman AW, et al. Calcium and calpain as key mediators of apoptosis-like death induced by vitamin D compounds in breast cancer cells. J Biol Chem 2002;277:30738-30745.
- 35. Schwarz EC, Qu B, Hoth M. Calcium, cancer and killing: the role of calcium in killing cancer cells by cytotoxic T lymphocytes and natural killer cells. Biochim Biophys Acta 2013;1833:1603-1611.
- 36. Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol 2001;2:316-324.
- 37. Bendsen NT, Hother AL, Jensen SK, Lorenzen JK, Astrup A. Effect of dairy calcium on fecal fat excretion: a randomized crossover trial. Int J Obes 2008;32:1816-1824.
- 38. Chang HH, Moro A, Takakura K, Su HY, Mo A, et al. Incidence of pancreatic cancer is dramatically increased by a high fat, high calorie diet in KrasG12D mice. PLoS One 2017;12:e0184455.
- 39. Zyromski NJ, Mathur A, Pitt HA, Wade TE, Wang S, et al. Obesity potentiates the growth and dissemination of pancreatic cancer. Surgery 2009;146:258-263.
- 40. Friling M, Haber A, Furman-Assaf S, Israel D, Harari G, et al. Bioavailability of calcium in an enriched postbiotic system compared to calcium citrate in healthy postmenopausal females; a randomized, double-blind, comparator-controlled, crossover study. Front Nutr 2023;10:1073622.
- 41. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. In Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds, ddDietary reference intakes for calcium and vitamin D. Washington, D.C.: National Academies Press; 2011.
- 42. Hammer GP, du Prel JB, Blettner M. Avoiding bias in observational studies: part 8 in a series of articles on evaluation of scientific publications. Dtsch Arztebl Int 2009;106:664-668.
- 43. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 2000;53:1119-1129.
 , Milad Mohammadzadeh2
, Milad Mohammadzadeh2 , Fatemeh Abdi3
, Fatemeh Abdi3 , Amin Paydareh2
, Amin Paydareh2 , Saman Khalesi4
, Saman Khalesi4 , Ehsan Hejazi2
, Ehsan Hejazi2