ABSTRACT
Obesity is a significant global health concern that not only increases metabolic disorders risks but also impacts mental health, particularly affecting women due to hormonal fluctuations and societal pressures. This study investigated anti-obesity and anti-anxiety effects of lemon balm (Melissa officinalis) extracts in female C57BL/6 mouse (n = 16, 17 weeks old) fed a high-fat diet (HFD). We compared 2 extracts method: distilled water (LBD, n = 5) and 80% ethanol (LBE, n = 6), administered via oral gavage (200 mg/kg/day) for 6 weeks alongside HFD. Both extract groups showed lower weight increase ratio compared to the control group in experiment period (n = 5) (LBD: 27.74%, LBE: 29.71% vs. Control: 51.88%, p < 0.05). The extracts significantly decreased mesenteric white adipose tissue (mWAT) among WATs examined (mWAT and parametrial white adipose tissue [pWAT]). While both LBD and LBE reduced fatty acid synthase (FAS) mRNA expression in pWAT, only LBD reduced peroxisome proliferator-activated receptor gamma and FAS mRNA expression in mWAT. In elevated plus maze behavioral experiments, the LBD group displayed reduced anxiety-like behavior, spending significantly more time and travelling greater distances in the open arms compared to other groups (p < 0.05), independent of brain inflammatory markers. Our findings demonstrate lemon balm extracts simultaneously address both obesity and anxiety-like behaviors in female mice, with extraction solvent-dependent variations in efficacy and mechanism of action. These results suggest potential therapeutic applications for lemon balm as a functional food ingredient, particularly for women experiencing concurrent obesity and anxiety symptoms.
-
Keywords: Lemon balm; Melissa officinalis; Obesity; Anxiety; Female; Extraction solvent
INTRODUCTION
The global prevalence of overweight and obesity continues to rise, making the regulation of excessive fat accumulation in the body an important challenge [
1,
2]. The World Health Organization has established guidelines to determining the presence of overweight and obesity. Individuals with a body mass index (BMI) of 25 kg/m
2 or higher as overweight, while those with a BMI of 30 kg/m
2 or higher are designated as obese [
3]. However, in Asia, the criterion for obesity is based on a BMI of 25 kg/m
2 or higher, which can result from a combination of excessive energy intake, genetic factors, irregular eating habits, and low physical activity [
4]. The persistence of these conditions can increase the risk of several diseases, including type 2 diabetes, cancer, cardiovascular diseases, and major depressive disorder (MDD) [
5]. This disruption to the body’s homeostasis underscores the imperative for timely management, emphasizing the need for effective interventions to mitigate these risks.
MDD is estimated to affect approximately 46 million people worldwide as of 2020 [
6]. Like obesity, MDD can emerge from multiple etiologies, including genetics and dietary habits. Prolonged symptoms can have a detrimental impact on daily life and potentially lead to suicide, thus necessitating proper management to alleviate symptoms [
7]. Recent studies have indicated a significant correlation between MDD and gut microbiota, suggesting that changes in gut microbiota caused by obesity resulting from high-fat diets (HFDs) can exacerbate feelings of depression [
8].
In South Korea, the number of patients diagnosed with depression increased by approximately 250,000 from 2018 to 2022, with the highest rates observed in women in their 20s in 2022 [
9]. It is noteworthy that women generally exhibit a lower sensitivity to depression, and the reasons for the high rates of depression among young women remain unclear [
10]. One hypothesis suggest that women’s higher body fat levels, societal pressures regarding appearance, and the increasing prevalence of obesity might contribute to their heightened risk of mood disorders like depression [
11]. From 2012 to 2021, the obesity rate among women age 20–29 rose by 87.63%, suggesting that this rise in obesity may be linked to the increased prevalence of depression in young women [
4].
Lemon balm, an herb primarily consumed as tea or used in oil form, is known for its calming effects [
12]. However, recently it has garnered attention for its potential anti-obesity properties, often when combined with other extracts, with rosmarinic acid identified as the primary component responsible for these effects. Rosmarinic acid, a representative phenolic compound found in lemon balm, has been reported to possess not only anti-inflammatory and antioxidant properties but also anti-obesity characteristics [
13,
14,
15]. To confirm the anti-obesity effects of lemon balm combined extracts, previous study examined some key biomarkers associated with anti-obesity reflect various physiological changes related to fat reduction, including decreased fat mass, inhibition of fat digestion and absorption, suppression of fat synthesis, promotion of fat breakdown, and stimulation of energy expenditure. Lemon balm combined with other extract showed significant changes in the expression of genes involved in adipocyte differentiation and fatty acid synthesis, fatty acid oxidation, such as Adiponectin, C/EBPα, C/EBPβ, Leptin, peroxisome proliferator-activated receptor (PPAR)-α, PPAR-γ, SREBP-1c, and UCP2, when lemon balm was consumed alongside other extracts [
14].
Therefore, this study aimed to investigate whether lemon balm alone could exert anti-obesity effects by inhibiting adipocyte differentiation and fat synthesis while promoting energy expenditure through thermogenesis. To this end, lemon balm extracted by distilled water and 80% ethanol. Distilled water was chosen as a safe solvent to extract hydrophilic compounds, while 80% ethanol, with a relative polarity of 0.710, was selected to effectively extract non-polar compounds and low-molecular-weight compounds that are not extractable with distilled water [
16].
Parametrial white adipose tissue (pWAT) located near the reproductive organs and mesenteric white adipose tissue (mWAT) located in the mesentery are visceral WAT. But, pWAT is closely related to reproductive functions, playing a role in hormonal regulation and energy provision for reproduction, whereas mWAT is known to act as a physical barrier against inflammation and regulate immune responses to the translocation of gut bacteria [
17,
18]. Due to these functional differences, it was hypothesized that lemon balm extracts would have different effects on the 2 types of fat tissue. Therefore, this study compared the effects of lemon balm on pWAT and mWAT to elucidate its potential as an independent anti-obesity agent and its underlying mechanisms. Additionally, the expression of PPAR-γ coactivator 1-alpha (PGC1-α) in brown adipose tissue (BAT) was examined to determine whether lemon balm exerts anti-obesity effects through thermogenesis.
Thus, in this study we will assess the anti-obesity and anti-anxiety effects of lemon balm extract alone, with the objective of exploring its potential as a functional food ingredient that may benefit young women struggling with obesity and depression.
MATERIALS AND METHODS
Preparation of lemon balm extracts
The lemon balm extract used in the experiment was derived from commercially available lemon balm tea bags. For extraction process, distilled water and 80% ethyl alcohol were prepared. Initially, the lemon balm from the tea bags was ground into a powder using a grinder. Then an extraction solvent equivalent to 10 times the weight of the powder was added, and the mixture was incubated in a shaking incubator at 60°C, 150 rpm for 5 hours. Upon completion of the incubation, the mixture was briefly cooled to room temperature, followed by centrifugation at 3,000 × g for 30 minutes. The supernatant was then filtered using what man filter paper. Subsequently, the extract was concentrated using a rotary evaporator, and finally dried for 24 hours using a freeze dryer before being stored at −80°C.
Animals and treatments
The experimental animals were approved by the Animal Experiment Ethics Committee of Chungnam National University (202410A-CNU-204), and a total of 16 female C57BL/6 mice aged 17 weeks were used. The experimental groups were randomly assigned as follows: HFD (Control) (n = 5), HFD + lemon balm distilled water extract (LBD) (n = 5), and HFD + lemon balm 80% ethanol extract (LBE) (n = 6). The animal housing facility was maintained at a temperature of 25°C ± 2°C, a humidity of 55% ± 5%, and a 12-hour light-dark cycle. Mice had free access to water and food (D12492 [60% fat]). Over a period of 6 weeks, a total of 200 mg/kg of the extract was administered daily via oral gavage (
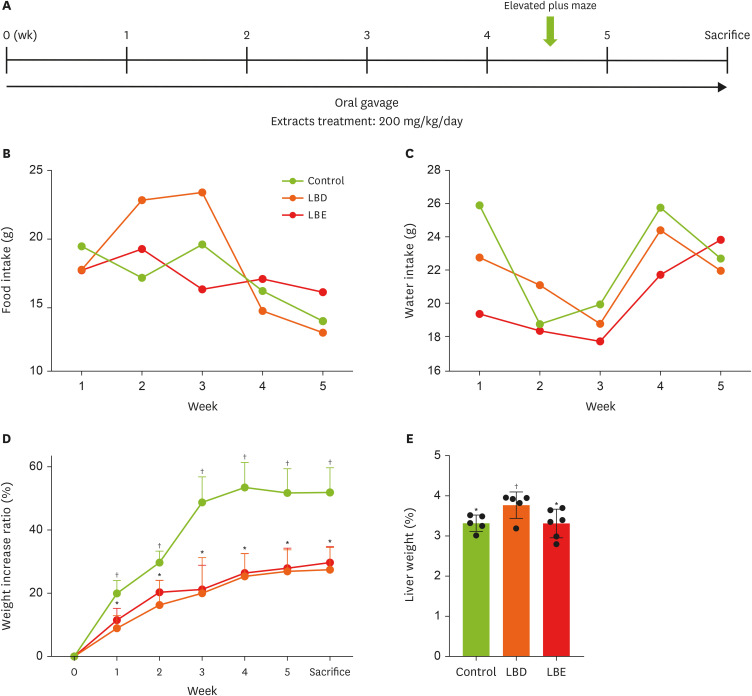
Figure 1A). For the group consuming only the HFD, phosphate-buffered saline was administered via oral gavage instead. After sacrifice, tissues used in the experiment were collected, weighed, and snap-frozen in liquid nitrogen before being stored at −80°C.
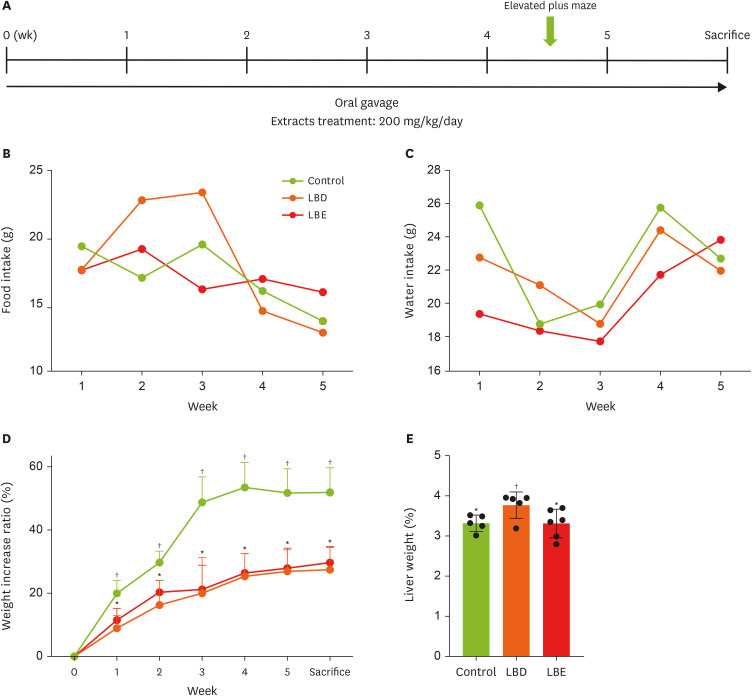
Figure 1
Basic information of experimental mouse. (A) The timeline of the experiment. (B, C) The consumption of food and water. (D) The changes of weight increase ratio in experimental period. (E) The liver weight which collected after sacrifice. The weight increase rate during the experiment period was expressed as a ratio of the amount increased compared to 0 week after the first weight measurement date was base line, and fat weight was calculated to the extent that the weight ratio of fat to weight was calculated, reducing the error in fat weight according to mouse size. Results were represented as mean ± standard deviation. Statistical significance was determined using one-way analysis of variance followed by Duncan’s multiples range tests.
Control, high-fat diet; LBD, high-fat diet + lemon balm distilled water extract; LBE, high-fat diet + lemon balm 80% ethyl alcohol extract.
*,†Different symbols indicated significant differences (p < 0.05). LBD and LBE share same letter in linear graph.
Blood analysis
To access the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) in serum, we used measurement kits (Cat No. AM103-K, AM102; ASAN Pharm, Seoul, Korea). The color intensity was measured within 60 minutes at 505 nm using an ELISA reader (microplate absorbance spectrophotometer; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
RNA extraction & cDNA synthesis
TRI reagent (MRC Inc., Cincinnati, OH, USA) was used for RNA extraction. Chloroform (Junsei Co., Tokyo, Japan) was subsequently added, and the homogenate was subjected to centrifugation at 12,000 × g for 15 minutes at 4°C. The supernatant was collected and subsequently treated with isopropanol (Duksan Co., Ansan, Korea). The mixture was then subjected to centrifugation at 12,000 × g for 8 minutes at 20°C. The resulting pellet was then removed, and the RNA concentration from the pellet was quantified using a NanoDrop spectrophotometer (Thermo Scientific Inc., Waltham, MA, USA). The cDNA was synthesized using the RT Kit (Biofact Co., Daejeon, Korea).
Reverse transcription polymerase chain reaction (RT-PCR) & real time transcription polymerase chain reaction (qPCR)
In order to quantify the mRNA expression of fatty acid synthase (FAS), PPAR-γ, tumor necrosis factor-α, interlukin-6 (IL-6), PGC1-α. RT-PCR was performed using 2× Taq Basic PCR Master Mix according to the manufacturer’s instructions (Biofact Co.). The samples were loaded on to 1.5% and 2% agarose gels for electrophoresis and observed using a gel documentation system (AE-9000 E-Graph; ATTO Co., Tokyo, Japan) under UV light. In qPCR, mRNA expression levels were assessed by AriaMx1.0 Real Time PCR system (Agilent Co., Santa Clara, CA, USA) using the 2× Real-Time PCR Master Mix (Including SYBR Green I, Low ROX; Biofact Co.). The primer sequences used are detailed in
Table 1.
Table 1Reverse transcription polymerase chain reaction and real time transcription polymerase chain reaction primer sequence
Table 1
|
Gene |
Primer |
Sequence (5’ → 3’) |
|
mGAPDH |
Fw |
ACAACTTTGGCATTGTGGAA |
|
Rv |
GATGCAGGGATGATGTTCTG |
|
mIL-6 |
Fw |
CCGGAGAGGAGACTTCACAG |
|
Rv |
GGAAATTGGGGTAGGAAGGA |
|
mTNF-α |
Fw |
CTCTTCAAGGGACAAGGCTG |
|
Rv |
GGACTCCGCAAAGTCTAAG |
|
mFAS |
Fw |
TGCTCCCAGCTGCAGGC |
|
Rv |
GCCCGGTAGCTCTGGGTGTA |
|
mPPAR-γ |
Fw |
GTCTGTGGGGATAAAGCATC |
|
Rv |
CTGATGGCATTGTGAGACAT |
|
mβ-actin |
Fw |
TAC CAC CAT GTA CCC AGG CA |
|
Rv |
CTC AGG AGG AGC AAT GAT CTT GAT |
|
mPGC1-α |
Fw |
AAG TGT GGA ACT CTC TGG AAC TG |
|
Rv |
GGG TTA TCT TGG TTG GCT TTA TG |
Western blotting
After homogenizing tissues in RIPA buffer using a GentleMACS™ Dissociator (Miltenyi Biotec Co., Bergisch Gladbach, Germany), the protein concentrations were measured using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific Inc., Rockford, IL, USA). Protein lysates were dissolved in electrophoresis sample buffer and boiled for 5 minutes at 100°C. Proteins were separated in polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were then blocked with 5% skim milk in Twin Tris-buffered saline (TTBS) and incubated overnight at 4°C with primary antibodies. The primary antibodies used were PGC1-α (Abcam, Cambridge, UK) and β-actin (Cell Signaling Technology, Danvers, MA, USA). Membranes were then washed with 1× TTBS 3 times and incubated with secondary goat anti-rabbit antibody (1:10,000; Invitrogen, Waltram, MA, USA). Visualization was performed by chemiluminescence Western blot reagents (Thermo Fisher Scientific Inc., Carlsbad, CA, USA) and ChemiDoc system (ATTO Co.).
Elevated plus maze (EPM)
The EPM experiment involved a cross-shaped maze set at a specific height above the ground, with 2 arms enclosed by walls and 2 open arms. The experiment lasted for 5 minutes, measuring the time and distance the mice spent in the open versus enclosed arms to assess anxiety. The experiments were recorded on video and analyzed using the SMART Video Tracking software from PanLab/Havard Apparatus, or the measurements taken by 3 individuals were averaged for analysis.
Statistical analysis
Data was analyzed using SPSS 24.0 (SPSS Inc., Chicago, IL, USA) software. Results are displayed as the mean ± standard deviation. A statistical analysis of the comparison between the 2 groups was performed using an independent t-test. One-way analysis of variance by Duncan multiples range tests was used to analyze 3 or more groups. Statistical significance was determined at p < 0.05.
RESULTS
Effects of lemon balm extracts in food, water intake and weight regulation
In this study, to assess the changes in basic parameters caused by extract consumption, the food intake, water intake, and weight gain rate of the mice were monitored weekly to determine whether changes in food and water intake influenced their body weight. The results revealed no significant differences in food or water intake among the groups; however, disparities in weight changes relative to the initial weight began to emerge by the end of the first week of the experiment (
Figure 1B-D). In the first week, the control group exhibited a weight gain rate of 19.92% relative to their initial weight, whereas the LBD group showed a weight gain rate of 8.93%, and the LBE group displayed a 11.49% increase. The differences in weight gain rates between the control group and the LBD and LBE groups remained significant until the time of sacrifice. On the day of sacrifice, the control group demonstrated a weight gain rate of 51.88% compared to their initial weight, while the LBD and LBE groups showed respective increases of 27.44% and 29.71%. These finding suggest that the consumption of lemon balm extract resulted in a reduction of weight gain rates compared to the control group (
Figure 1D). However, no significant differences were observed in weight gain rates between the LBD and LBE groups. Prolonged exposure to HFD or excessive administration of the extract has potential to result in non-alcoholic fatty liver disease (NAFLD) and extract-related liver toxicity. Therefore, this study measured liver weight and serum AST and ALT levels to investigate this potential issue (
Figures 1E and
2D). The results indicated a significant increase in liver weight in the LBD group. However, serum AST and ALT levels did not show significant differences across all groups. Consequently, at efforts was made to determine if an inflammatory response occurred in the liver via RT-PCR (
Figure 2C).
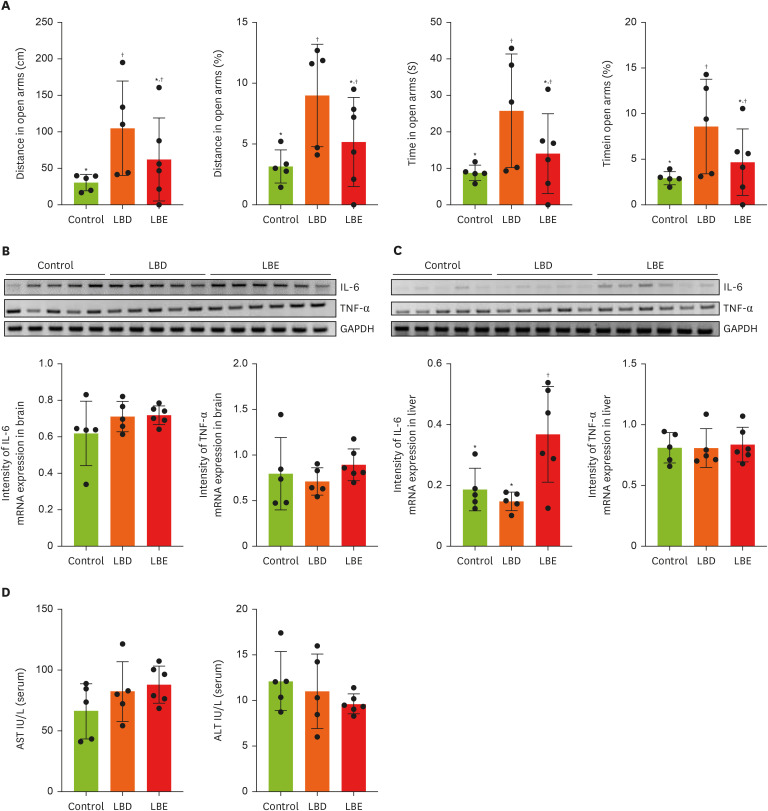
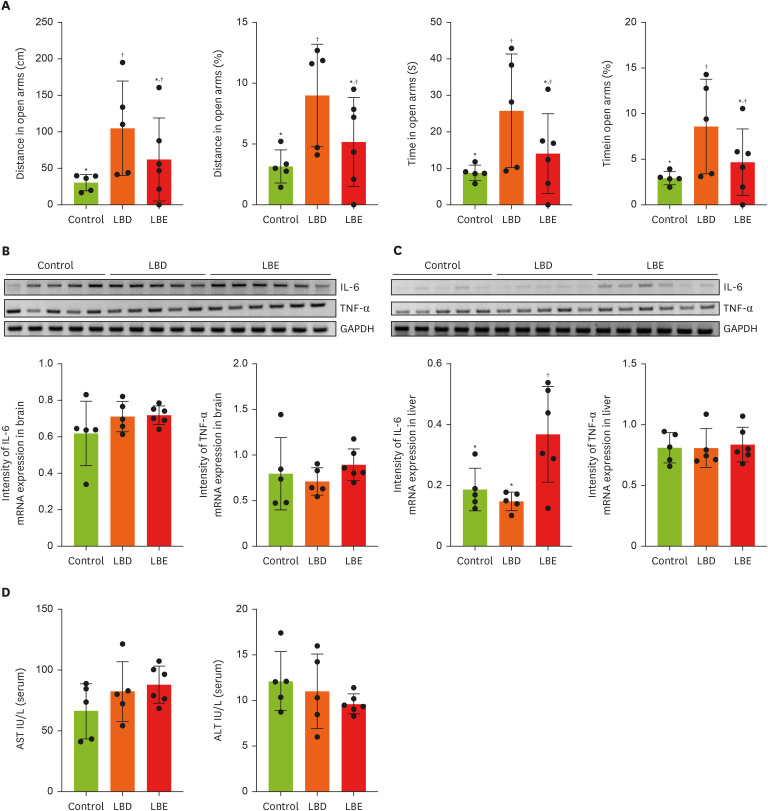
Figure 2
Effect of Lemon balm extracts on anxiety-like behavior change and expression level of IL-6 and TNF-α in brain and liver. To access anti-anxiety effects of extracts, elevated plus maze was conducted. (A) The results of traveling time and distance in open arms. (B, C) The expression level of IL-6 and TNF-α in brain and liver by gel image and display graphs illustrating quantification of images obtained through gel electrophoresis using Image J. Graphic images were processed and analyzed with Image J to quantify the data, and the results are presented in graphical form. (D) The amount of AST and ALT in serum. Results were represented as mean ± standard deviation. Statistical significance was determined using one-way analysis of variance followed by Duncan’s multiples range tests.
Control, high-fat diet; LBD: high-fat diet + lemon balm distilled water extract; LBE, high-fat diet + lemon balm 80% ethyl alcohol extract; TNF-α, tumor necrosis factor-α; IL-6, interlukin-6; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
*,†Different symbols indicated significant differences (p < 0.05).
Effects of lemon balm extracts in adipose tissue metabolism
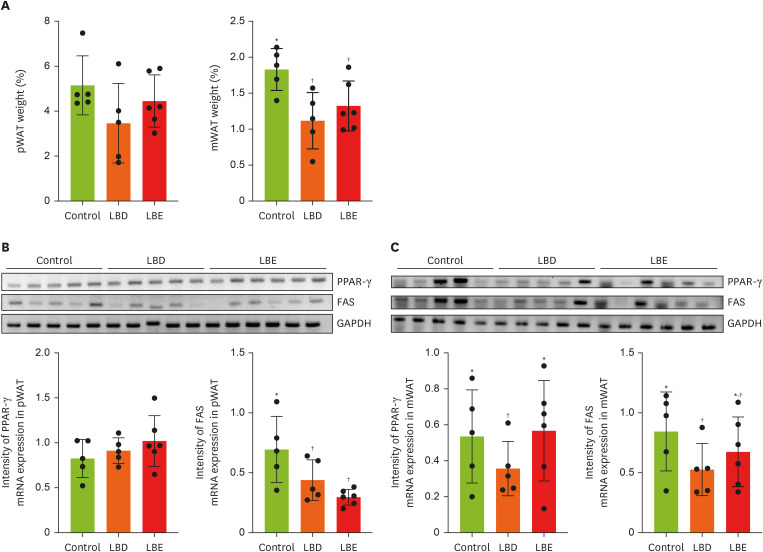
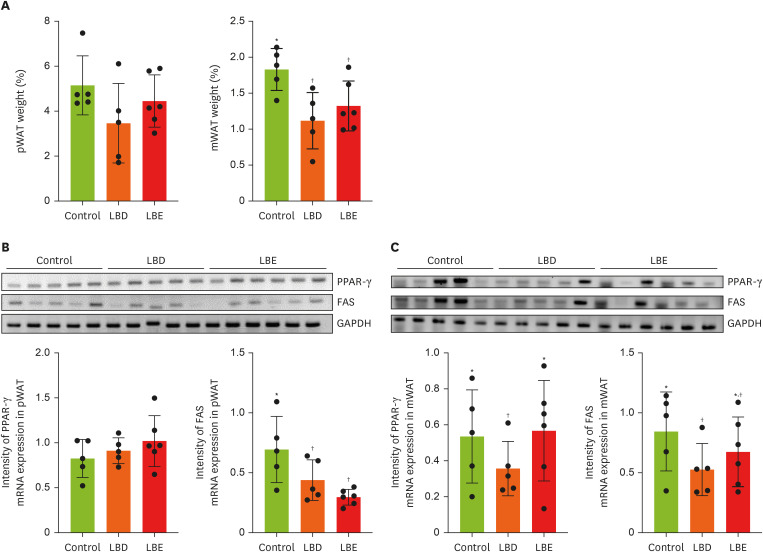
To determine the potential inhibitory effects of lemon balm extract on WAT synthesis and differentiation, we analyzed pWAT and mWAT weights (
Figure 3A), along with expression levels of genes related to fat synthesis (FAS) and adipocyte proliferation regulation (PPAR-γ) using RT-PCR (
Figure 3B and C). While no statistically significant differences were observed in pWAT weight among groups, both extract group exhibited comparatively reduced mWAT weight compared to the control group. Although the absence of significant pWAT weight reduction, both LBD and LBE groups showed downregulation of FAS expression. Notably, only LBD group exhibited downregulation of PPAR-γ and FAS in mWAT, despite both extract groups’ lower mWAT weight.
Figure 3
Effects of lemon balm extracts in WAT weight regulation and expression level of PPAR-γ and FAS. To access anti-obesity effects of extracts, PPAR-γ and FAS mRNA level were measured by reverse transcription polymerase chain reaction. GAPDH was used for control. (A) The tissue weight which collected after sacrifice. (B, C) The expression level of PPAR-γ and FAS in pWAT and mWAT by gel image and display graphs illustrating quantification of images obtained through gel electrophoresis using Image J. Graphic images were processed and analyzed with Image J to quantify the data, and the results are presented in graphical form. Results were represented as mean ± standard deviation. Statistical significance was determined using one-way analysis of variance followed by Duncan’s multiples range tests.
Control, high-fat diet; LBD, high-fat diet + lemon balm distilled water extract; LBE, high-fat diet + lemon balm 80% ethyl alcohol extract; pWAT, parametrial white adipose tissue; mWAT, mesenteric white adipose tissue; PPAR-γ, peroxisome proliferator-activated receptor gamma; FAS, fatty acid synthase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
*,†Different symbols indicated significant differences (p < 0.05).
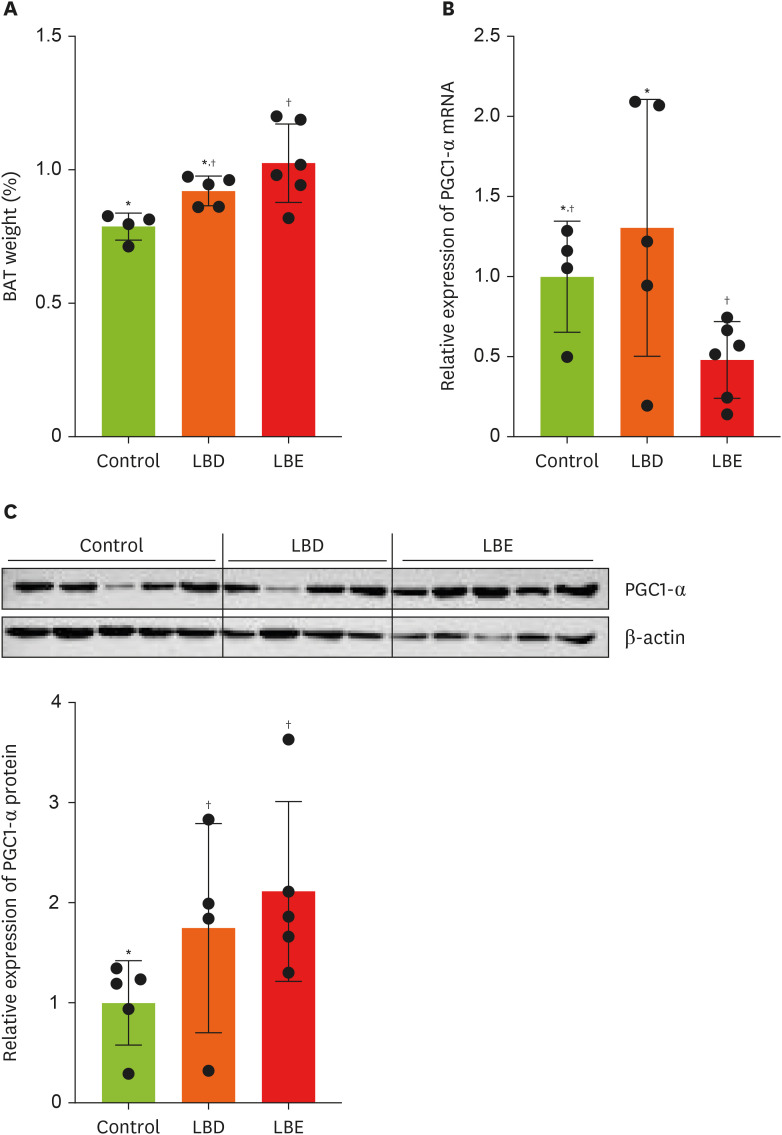
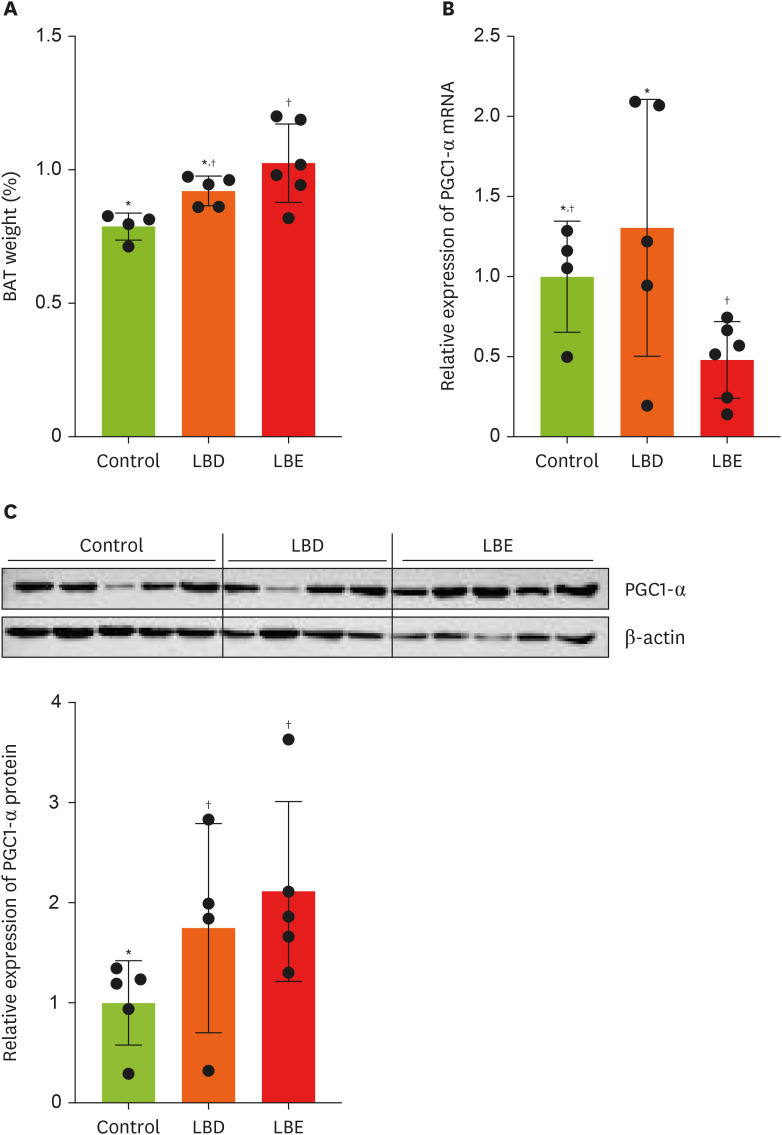
In order to ascertain additional anti-obesity impacts beyond WAT mass reduction, we compared BAT weights among the groups (
Figure 4A). The LBE group exhibited a significant increased BAT weight compared to the control group. As a result, we analyzed the expression of PGC1-α, a key regulator of mitochondrial biogenesis and anti-obesity effects in BAT, using qPCR [
19]. The analysis revealed decreased PGC1-α mRNA expression in the LBE group compared to the LBD group (
Figure 4B). Interestingly, Western blot analysis revealed that PGC1-α protein expression in the LBE group was elevated compared to the control group, contrasting with the qPCR results (
Figure 4C).
Figure 4
Effects of lemon balm extracts in BAT weight regulation and expression level of PGC1-α. (A) The tissue weight which collected after sacrifice. (B) The expression level of PGC1-α in BAT. (C) The expression level of PGC1-α protein level in BAT. Graphic images were processed and analyzed with Image J to quantify the data, and the results are presented in graphical form. Results were represented as mean ± standard deviation. Statistical significance was determined using one-way analysis of variance followed by Duncan’s multiples range tests.
Control, high-fat diet; LBD, high-fat diet + lemon balm distilled water extract; LBE, high-fat diet + lemon balm 80% ethyl alcohol extract; BAT, brown adipose tissue; PGC1-α, peroxisome proliferator-activated receptor gamma coactivator 1-α.
*,†Different symbols indicated significant differences (p < 0.05).
Lemon balm improved anxiety like behavior in EPM, without regulation of brain & liver inflammation
Inflammation is an essential immune response within the body, crucial for maintaining tissue and mechanism homeostasis by clear the inflammatory triggers [
20]. However, excessive inflammatory responses triggered by external stimuli or endogenous factors can disrupt this balance, leading to further organ damage [
21]. Symptoms related to mental health, such as anxiety and depression, can arise from various causes, but one contributing factor may be brain inflammation induced by excessive stress responses caused by obesity or HFD exposure [
22,
23]. In this study, we aimed to investigate the effects of lemon balm extract on anxiety caused by a HFD through behavioral experiments [
12].
We also sought to determine whether the extract regulates brain inflammation to alleviate anxiety and depressive like behaviors. Additionally, to assess whether the extract itself could cause liver inflammation due to toxicity, we examined the expression levels of inflammatory cytokines in the liver using RT-PCR [
24]. In the EPM test, which measures anxiety by assessing the time spent and distance traveled in the open arms and closed arms, the LBD group showed a significant increase in both time spent and distance traveled in the open arms compared to the control group (
Figure 2A). However, no differences were observed between the LBE group and the control or LBD groups. To confirm whether this anxiolytic effect was due to a reduction in brain inflammation, we compared the expression levels of inflammatory cytokines through RT-PCR, but no significant differences were found between the groups in terms of cytokine expression (
Figure 2B). Lastly, to assess the potential impact of the extract on liver inflammation, we examined the expression levels of inflammatory cytokines in the liver (
Figure 2C). The results showed that the IL-6 expression in the LBE group was higher compared to the other groups.
DISCUSSION
Our study demonstrates that lemon balm extract effectively reduces weight gain and anxiety-like behaviors in female mice fed a HFD, with varying effects depending on the extraction solvent used. Here, we discuss the underlying mechanisms, implications, and future directions of these findings.
The anti-obesity effects of lemon balm extracts manifested through distinct mechanisms depending on the extraction method. Water extract (LBD) primarily acted by suppressing adipogenesis, as evidenced by reduced expression of FAS and PPAR-γ in WAT. This suggests that water-soluble components, such as rosmarinic acid, of lemon balm may directly influence lipid metabolism and adipocyte differentiation pathways. In contrast, ethanol extract (LBE) demonstrated its anti-obesity effects primarily through increased BAT mass and enhanced thermogenic capacity, despite minimal impact on WAT-related gene expression [
25].
The increased size of BAT in the LBE group is likely related to its thermogenic effects, as BAT contains a high density of mitochondria, which promote ATP production and induce thermogenesis through uncoupling protein 1 expression [
26]. These thermogenic effects contribute to the anti-obesity benefits observed in the LBE group. However, while the LBE group exhibited similar effects on weight gain reduction as the LBD group, its influence on mRNA expression related to weight gain in WAT was weaker. This discrepancy highlights the need to understand the mechanisms underlying the observed BAT enlargement.
To further investigate, the study measured PGC1-α using qPCR and Western blot analysis. As a result, LBD and LBE group didn’t showed the increase PGC1-α mRNA level. However, qPCR revealed that the mRNA expression of PGC1-α was lower in the LBE group compared to the LBD group. But in Western blot analysis showed that PGC1-α protein levels were significantly higher in the LBD and LBE group. Interestingly, unlike LBD group, LBE group showed unmatched results between qPCR and Western blot. This divergence between mRNA and protein expression can be explained by post-transcriptional regulatory mechanisms. mRNA is first transcribed and serves as the template for protein synthesis. Once sufficient protein levels are reached, feedback mechanisms may downregulate mRNA expression to conserve cellular resources [
27]. Thus, the elevated protein levels of PGC1-α in the LBE group likely reflect an active thermogenic state, even as mRNA levels decrease due to regulatory feedback.
These findings align with studies that report mismatches between mRNA and protein levels. For example, Gygi et al. [
28] and Schwanhäusser et al. [
29] demonstrated that protein abundance is influenced not only by mRNA availability but also by translation rates and protein stability. This suggests that the higher protein levels of PGC1-α in the LBE group may be responsible for the increased BAT size and associated thermogenic effects as like LBD group, despite the reduced mRNA expression.
It is acknowledged that depression and anxiety behaviors arise from various factors, including hormones and neuro-inflammation, rather than a single cause. Based on previous research findings, we noted that the secretion of pro-inflammatory cytokines from adipocytes due to obesity could increase brain inflammation, and we hypothesized that HFD might be linked to increased anxiety behaviors [
21,
22]. Regarding behavior test, our study revealed that only LBD treatment resulted in significant anxiolytic effects, as demonstrated by increased exploration in the EPM test. Interestingly, this behavioral improvement occurred independently of changes in neuroinflammatory markers, suggesting that LBD’s anxiolytic effects may operate through alternative pathways, and indicates the need to explore other potential mechanisms, such as neurotransmitter systems or hormonal regulation.
Several limitations of our study should be acknowledged. First, the relatively small sample size (n = 5–6 per group) may limit the statistical power of our findings. Second, our behavioral assessment relied solely on the EPM test; additional behavioral tests such as the open field test or forced swim test would provide more comprehensive evaluation of anxiety- and depression-like behaviors. And last, contrary to our expectation that the control group, which showed greater weight gain, would exhibit increased levels of ALT and AST along with higher expression of inflammatory cytokines because of NAFLD, but there were no significant differences in serum ALT and AST levels were observed between the groups and also LBE group showed higher expression level of IL-6 in liver. This suggests that the mice used in the experiment may not have developed NAFLD, or the inflammation caused by excessive weight gain was not sufficiently induced to significantly cellular damage to liver. Case of LBE group, which exhibited increased expression of inflammatory cytokine IL-6 in liver compared to the other groups, despite the differences in ALT and AST levels, increased IL-6 expression in the liver may indicate the possibility of the presence of local inflammation in the absence of obvious hepatocellular damage. ALT and AST are indicators of hepatocellular damage, whereas IL-6 is a pro-inflammatory cytokine that can be elevated by inflammation regardless of immune cell activity or apparent cell damage. This suggests that the inflammatory response of the liver is in its early stages, or that it is likely confined to immune activity rather than progressing to widespread hepatocellular damage detectable by ALT and AST.
A notable finding in this study was the differential effects observed based on extraction solvent. Despite employing identical extraction methodologies, the biological effects varied significantly with different solvent. Although both extracts showed comparable effects on body weight reduction, the LBE group demonstrated no improvement in anxiety-related behavior. Moreover, the LBE group exhibited increased expression of inflammatory cytokine IL-6 in liver compared to the control group, suggesting potential liver inflammation.
These differential effects can be attributed to variation in type and extraction efficiencies of compounds depending on the solvent used during plant extraction. Usually, ethanol extraction has the advantage of efficiently extracting both polar and non-polar compounds, while water, although considered the safest solvent and widely used for extracting water-soluble components, is known to have relatively lower extraction efficiency [
30]. Therefore, it is thought that the types and amounts of compounds contained in the extracts were different in LBE and LBD, so the difference would have been as shown in the results of this study, and this result could be a basis for recommending the extraction solvent used in the future lemon balm extraction. However, limitation of the present study is lack of compositional analysis of the extracts. It is believed that an additional process is needed to verify the amount and types of compounds according to the extraction solvent.
CONCLUSION
In conclusion, our study provides evidence that lemon balm extracts can simultaneously address both obesity and anxiety-like behaviors, with water extract showing particular promise through its dual effectiveness. These findings support the potential development of lemon balm-based interventions for women experiencing concurrent obesity and anxiety symptoms, although further research is needed to fully understand the mechanisms of action and optimize therapeutic applications. Future studies should focus on identifying specific bioactive compounds, establishing optimal extraction methods, and conducting carefully designed clinical trials to validate these promising preliminary findings.
Ministry of Education of the Republic of Koreahttps://doi.org/10.13039/501100002701
National Research Foundation of Koreahttps://doi.org/10.13039/501100003725
NRF-2022R1A2C1091570
NOTES
-
Funding: This research was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2022R1A2C1091570).
-
Conflict of Interest: The author declares that they have no competing interests.
-
Author Contributions:
Conceptualization: Cho J.
Formal analysis: Kim JG.
Investigation: Cho J.
Methodology: Kim JG, Park J, Baek S, Won S.
Supervision: Kim JG.
Writing - original draft: Kim JG.
Writing - review & editing: Kim J, Cho J.
REFERENCES
- 1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017;390:2627-2642.
- 2. GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13-27.
- 3. World Health Organization (WHO) Consultation on Obesity. Obesity: preventing and managing the global epidemic: report of a WHO consultation. Geneva: WHO; 2000.
- 4. Korean Society for the Study of Obesity. Obesity fact sheet. Seoul: Korean Society for the Study of Obesity; 2023.
- 5. Pi-Sunyer X. The medical risks of obesity. Postgrad Med 2009;121:21-33.
- 6. World Health Organization (WHO). Depression and other common mental disorders. Geneva: WHO; 2017.
- 7. Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health 2013;34:119-138.
- 8. Schachter J, Martel J, Lin CS, Chang CJ, Wu TR, et al. Effects of obesity on depression: a role for inflammation and the gut microbiota. Brain Behav Immun 2018;69:1-8.
- 9. Health Insurance Review & Assessment Service (HIRA). Mental health trends depression. Wonju: HIRA; 2023.
- 10. Kuehner C. Why is depression more common among women than among men? Lancet Psychiatry 2017;4:146-158.
- 11. Blaine B. Does depression cause obesity?: a meta-analysis of longitudinal studies of depression and weight control. J Health Psychol 2008;13:1190-1197.
- 12. Kennedy DO, Scholey AB, Tildesley NT, Perry EK, Wesnes KA. Modulation of mood and cognitive performance following acute administration of Melissa officinalis (lemon balm). Pharmacol Biochem Behav 2002;72:953-964.
- 13. Rui Y, Tong L, Cheng J, Wang G, Qin L, et al. Rosmarinic acid suppresses adipogenesis, lipolysis in 3T3-L1 adipocytes, lipopolysaccharide-stimulated tumor necrosis factor-α secretion in macrophages, and inflammatory mediators in 3T3-L1 adipocytes. Food Nutr Res 2017;61:1330096.
- 14. Cho IJ, Kim SE, Choi BR, Park HR, Park JE, et al. Lemon balm and corn silk extracts mitigate high-fat diet-induced obesity in mice. Antioxidants 2021;10:2015.
- 15. Abdellatif F, Begaa S, Messaoudi M, Benarfa A, Ouakouak H, et al. HPLC-DAD analysis, antimicrobial and antioxidant properties of aromatic herb Melissa officinalis L., aerial parts extracts. Food Anal Methods 2023;16:45-54.
- 16. Plaskova A, Mlcek J. New insights of the application of water or ethanol-water plant extract rich in active compounds in food. Front Nutr 2023;10:1118761.
- 17. Eder P, Adler M, Dobrowolska A, Kamhieh-Milz J, Witowski J. The role of adipose tissue in the pathogenesis and therapeutic outcomes of inflammatory bowel disease. Cells 2019;8:628.
- 18. Yano A, Liu S, Suzuki Y, Imai M, Mogi M, et al. Single-cell transcriptomic architecture and cellular communication circuits of parametrial adipose tissue in pregnant mice. Life Sci 2023;334:122214.
- 19. Halling JF, Pilegaard H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl Physiol Nutr Metab 2020;45:927-936.
- 20. Fioranelli M, Roccia MG, Flavin D, Cota L. Regulation of inflammatory reaction in health and disease. Int J Mol Sci 2021;22:5277.
- 21. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860-867.
- 22. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol 2006;27:24-31.
- 23. Beilharz JE, Maniam J, Morris MJ. Short-term exposure to a diet high in fat and sugar, or liquid sugar, selectively impairs hippocampal-dependent memory, with differential impacts on inflammation. Behav Brain Res 2016;306:1-7.
- 24. Stickel F, Seitz HK, Hahn EG, Schuppan D. Liver toxicity of drugs of plant origin. Z Gastroenterol 2001;39:225-232. 234-227.
- 25. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004;84:277-359.
- 26. Kajimura S, Spiegelman BM, Seale P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab 2015;22:546-559.
- 27. Müller-McNicoll M, Rossbach O, Hui J, Medenbach J. Auto-regulatory feedback by RNA-binding proteins. J Mol Cell Biol 2019;11:930-939.
- 28. Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 1999;19:1720-1730.
- 29. Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, et al. Global quantification of mammalian gene expression control. Nature 2011;473:337-342.
- 30. Lee JE, Jayakody JTM, Kim JI, Jeong JW, Choi KM, et al. The influence of solvent choice on the extraction of bioactive compounds from Asteraceae: a comparative review. Foods 2024;13:3151.