ABSTRACT
The present systematic review and meta-analysis aimed to investigate the effects of Nigella sativa (N. sativa) supplementation on liver enzymes levels including aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Relevant studies, published from inception up to January 2020, were searched through PubMed, Scopus and Google Scholar conducted on randomized controlled trials (RCTs) investigating the effect of N. sativa on serum AST and ALT levels. Meta-analysis was applied using a random-effects model. Eight studies met inclusion criteria (n=281 in the N. sativa and n = 279 in placebo group). This meta-analysis showed that N. sativa supplementation significantly reduced AST level (weighted mean difference [WMD], −8.11 IU/L; 95% confidence interval [CI], −13.6, −2.53; p = 0.004) with significant heterogeneity (I-squared, 95.9%; p < 0.001) while the decrease in ALT level was not statistically significant (WMD, −7.26 IU/L; 95% CI, −15.4, 0.04; p = 0.051) with significant heterogeneity (I-squared, 97.8%; p < 0.001). This meta-analysis suggests that N. sativa supplementation may improve AST levels and ALT levels, however more RCTs with larger sample size are needed to found effects of N. sativa on liver enzymes in patients with non-alcoholic fatty liver disease.
-
Keywords: Nigella sativa; Liver enzymes; Alanine aminotransferase; Aspartate aminotransferase
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a common disease that affects approximately 25% of the world population [
1] and it is a critical health issue due to the increasing prevalence of 20%–30% in developed countries and 10%–20% in developing countries [
2]. NAFLD may cause cardiovascular and hepatic morbidities and mortalities and is highly associated with metabolic syndrome components, especially insulin resistance [
1]. NAFLD is known as an inflammatory disease that increases aminotransferases levels through oxidative stress and inflammation [
3]. Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) measurements are the most important sensitive diagnostic methods in liver dysfunction in clinical diagnosis [
4]. There is no definitive medication for NAFLD and weight loss and physical activity are only recommended for the treatment of NAFLD [
5].
Nigella sativa (
N. sativa) seeds (
Ranunculaceae family) [
6] are used for centuries in traditional medicine in the Middle East, North America, and South Asia to improve health and treat some diseases such as diabetes, obesity, dyslipidemia, and hypertension [
7].
N. sativa has beneficial effects on NAFLD-related risk factors such as improving metabolic syndrome, blood pressure, weight gain, lipid profile in various studies [
8,
9,
10,
11], as well as having anti-inflammatory and antioxidant effects [
3]. Pharmacological studies showed that
N. sativa can be effective in treating NAFLD [
12]. It has been shown that
N. sativa has an agonistic role for peroxisome proliferator-activated receptor gamma (PPARγ), which these agonists reduce insulin resistance [
13]. Moreover,
N. sativa oil has been shown to affect lipid metabolism, especially hepatic triglycerides (TG) metabolism [
1].
Recent study found that supplementation with
N. sativa oil significantly reduced AST and ALT levels, TG, low density lipoprotein cholesterol (LDL-C) and steatosis degree, and significantly increased high density lipoprotein cholesterol (HDL-C) without affecting body weight [
12]. Other studies have also shown a significant reduction with
N. sativa supplementation at AST and ALT levels [
14,
15,
16], while one study has shown that
N. sativa supplementation has only significantly reduced ALT levels [
17]. Another study showed a significant increase in AST and insignificant increase in ALT [
18]. Given the inconsistencies about the effect of
N. sativa on liver enzymes, the present meta-analysis study aimed to evaluate the effect of
N. sativa supplementation on the liver enzymes.
MATERIALS AND METHODS
This meta-analysis addressing the effects of N. sativa supplementation on liver enzymes levels, was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement guidelines.
Search strategy
We systemically searched all RCTs in PubMed, Scopus and Google Scholar from inception to 15 January 2020. The following Medical Subject Heading (MeSH) and non-MeSH keywords were used to find relevant papers. The full search strategy is available in
Supplementary Table 1. We also hand-searched all the references from the included articles, to prevent missing any related research.
The search terms and strategies were constructed according to the PICOS model. Potentially relevant studies were included if they met the following inclusion criteria: conducting on adults aged > 18 years, having an intervention with N. sativa or thymoquinone, comparing with a control group, evaluating serum ALT and AST levels and with parallel and cross-over clinical trial design. Studies were excluded for those: (a) publications examining the effects of N. sativa supplementation in composition with other interventions such as lemon balm as a compound and its separate effect has not been investigated, (b) un-controlled trials, (c) animal or in vitro studies, and (d) studies without adequate data on liver enzymes.
Data extraction
The information was extracted independently by 2 researchers (Neda Azizi, Mohammad Reza Amini). Any dissimilarity and differences were resolved by third independent investigator (Sakineh Shab-Bidar), if necessary. Eligible studies were reviewed, and the following data were abstracted: first author's name, year of publication, study location, study design, trial duration and intervention (type and dose of supplementation); subjects' information, including mean of age, gender and number of participants in both groups (intervention and control), outcomes assessed, including baseline and final values of outcomes (ALT, AST).
Quality assessment
The revised Cochrane risk of bias tool (RoB 2) [
19] was used for quality assessment of the included studies with the following criteria: random sequence generation, allocation sequence concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other potential sources of bias. A judgment of low risk, high risk or unclear based on each item was made.
Meta-analysis was conducted using Stata software version 14 (Stata Corp., College Station, TX, USA). The pooled weighted mean difference (WMD) and its 95% confidence interval (CI) was used to assess the effects of
N. sativa supplementation on liver enzymes levels. The effect of
N. sativa on liver enzymes was defined as the mean difference of changes observed in the intervention group compared to the control group. Standard deviations of the mean difference were calculated using proper formula [
20].
Inter-study heterogeneity was assessed using I
2 index, with values > 50% as evidence of moderate to high heterogeneity. Random effects model by Der Simonian and Laird method was applied in the current meta-analysis [
21]. To investigate the sources of heterogeneity, a subgroup analysis of trial duration, intervention type, age, and sample size was performed. p value < 0.05 was considered to be statistically significant.
RESULTS
Search results and study selection
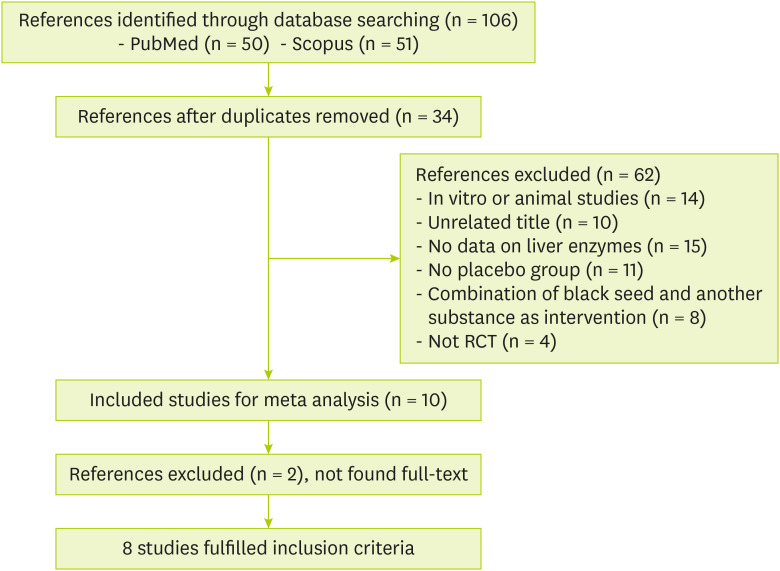
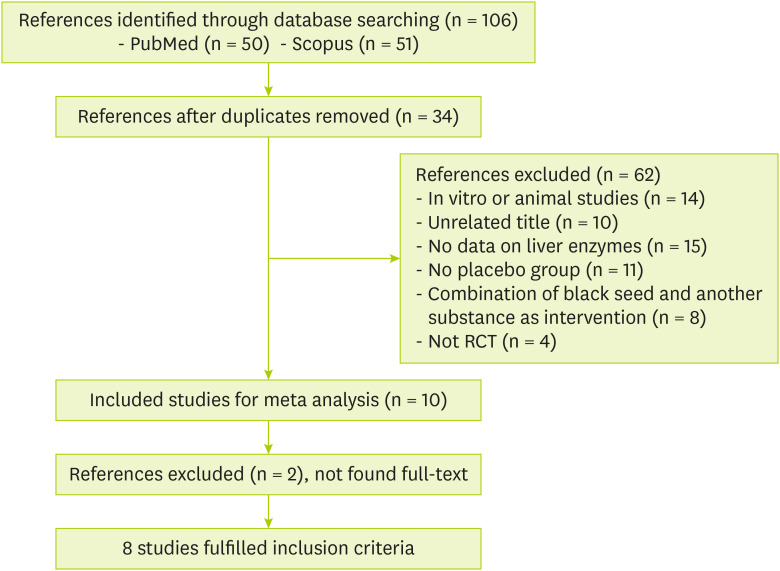
Out of 106 identified publications in the initial search, 34 duplicates were excluded. After screening by title and abstract, 28 studies were removed due to the primary evaluation of inclusion criteria: unrelated title (n = 10), animal study (n = 14), not RCTs (n = 4) and 44 papers were retained for the full-text review. Of these, 36 articles were excluded because of the following reasons: had no placebo-controlled group (n = 11), had combined intervention (n = 8), had no adequate data on liver enzymes (n = 15) and had no available full text (n = 2). Finally, 8 studies met all our inclusion criteria and included for the meta-analysis. The flow chart of the literature search has been shown in
Figure 1.
Figure 1
Flow diagram of the study.
RCT, randomized controlled trial.
Study characteristics
The characteristics of included studies were abstracted in
Table 1. Four studies were conducted in Iran [
12,
15,
16,
17], 3 in Pakistan [
14,
18,
22] and 1 in Indonesia [
23]. Selected eligible trials enrolled 519 participants with age range of 30 to 55 years old and were conducted on both gender. Publication dates ranged from 2009 to 2019. Four of the included studies were performed on NAFLD patients [
12,
14,
15,
16], while the remaining 4 were performed on people with different conditions, such as patients with diabetes [
17,
18,
22,
23]. Most studies have been performed on both genders except for 2 studies. [
17,
23]. Four studies used
N. sativa capsules [
14,
16,
22,
23] and 4 studies,
N. sativa oil [
12,
15,
17,
18].
Table 1Quality assessment of studies by the Revised Cochrane risk of bias tool (RoB 2)
Table 1
|
Study |
Random sequence generation |
Allocation concealment |
Blinding of participants and personnel |
Blinding of outcome assessments |
Incomplete outcome data |
Selective reporting |
Overall assessment of risk if bias |
|
Khonche et al. [15] |
L |
H |
H |
U |
L |
H |
U |
|
Hussain et al. [14] |
L |
H |
H |
H |
L |
L |
L |
|
Rashidmayvan et al. [16] |
L |
L |
L |
U |
L |
L |
L |
|
Khonche et al. [12] |
L |
L |
L |
U |
L |
L |
L |
|
Valizadeh et al. [17] |
L |
L |
L |
U |
L |
L |
U |
|
Datau et al. [23] |
L |
L |
L |
U |
L |
L |
U |
|
Ahmad et al.[18] |
U |
U |
U |
U |
L |
L |
L |
|
Qidwai et al. [22] |
L |
L |
L |
L |
L |
L |
L |
Quality assessment
All of studies had randomized design except for one [
18]. In 5 studies, participants did not know what group they were in and what they received [
12,
16,
17,
22,
23]. Blinding of outcome in most of studies was unclear [
12,
15,
16,
18,
23]. In all studies, the reports were balanced and both significant and non-significant results were shown except for one of them [
15]. Details of risk of bias assessment are described in
Table 2.
Table 2Studies of the effect of Nigella sativa supplementation on AST and ALT levels
Table 2
|
Author |
Location |
Study design |
Health status |
Gender (female/male) |
Sample size (male/female) |
Mean age |
Intervention |
Dose |
Mean BMI |
Control |
Outcome |
Duration (wk) |
|
Khonche et al. [15] |
Iran |
Randomized, double-blind, placebo-controlled trial |
NAFLD patients |
F/M |
64/56 |
47.92 ± 12.02 |
The N. sativa oil syrup |
2.5 mL oil/5 mL syrup |
27.88 ± 2.69 |
Placebo |
AST, ALT |
12 |
|
Hussain et al. [14] |
Pakistan |
Randomized, double-blind, placebo-controlled trial |
NAFLD patients |
F/M |
44/26 |
38.00 ± 8.75 |
The N. sativa cap |
1 g |
29.06 ± 4.60 |
Placebo |
AST, ALT |
12 |
|
Rashidmayvan et al. [16] |
Iran |
Randomized, double-blind, placebo-controlled trial |
NAFLD patients |
F/M |
29/15 |
39.00 ± 5.37 |
The N. sativa cap |
1 g |
27.59 ± 2.83 |
Placebo |
AST, ALT, GGT |
8 |
|
Khonche et al. [12] |
Iran |
Randomized, double-blind, placebo-controlled trial |
NAFLD patients |
F/M |
62/58 |
46.64 ± 12.18 |
The N. sativa oil syrup |
2.5 mL oil/5 mL syrup |
27.00 ± 2.10 |
Placebo |
AST, ALT |
12 |
|
Valizadeh et al. [17] |
Iran |
Randomized, double-blind, placebo-controlled trial |
Postmenopausal women with osteoporosis |
F |
12 |
55.00 ± 6.20 |
The N. sativa oil |
3 mL /0.05 mL/kg/d |
23.00 ± 5.00 |
Placebo |
AST, ALT, ALP |
12 |
|
Datau et al. [23] |
Indonesia |
Randomized, double-blind, placebo-controlled trial |
Central obese male |
M |
39 |
37.50 ± NA |
The N. sativa cap |
750 mg/3 times/d |
NA |
Placebo |
AST, ALT |
12 |
|
Ahmad et al. [18] |
Pakistan |
Randomized, double-blind, placebo-controlled trial |
Type 2 diabetes mellitus patients |
F/M |
17/24 |
47.80 ± 1.10 |
The N. sativa oil |
Oil of 0.7 g N. sativa
|
NA |
Placebo |
AST, ALT |
11.5 |
|
Qidwai et al. [22] |
Pakistan |
Randomized, double-blind, placebo-controlled trial |
Adults with hypercholesterolemia |
F/M |
73 (NA/NA) |
45.58 ± 10.86 |
The N. sativa cap |
500 mg/2 times/d |
27.13 ± 3.88 |
Placebo |
ALT |
6 |
Systematic review
Most studies have examined the effect of
N. sativa supplementation on ALT and AST levels [
12,
14,
15,
16,
17,
18,
23], except for one study that showed only the effect of
N. sativa supplementation on ALT level [
22]. Two studies in patients with NAFLD reported that receiving 5 mL of daily syrup containing 2.5 mL of
N. sativa oil compared to other patients with NAFLD who received placebo for 3 months significantly reduced ALT and AST levels. In first study, 2.5 mL mineral oil, 1.25 mL honey and 1.25 mL water in each 5 mL of the mixture as placebo and participants had low HDL and high AST and ALT and according to abdominal ultrasound, they had fatty liver in different degrees, finally, consumption of
N. sativa had a decreasing effect on LDL, TG and fatty liver grades while HDL increased. In second study, placebo syrup contained all the syrup additives except
N. sativa oil. People had low HDL and high liver enzymes at the beginning of the study and were overweight and in addition to liver enzymes, mean of LDL and TG reduced and HDL levels increased in intervention group [
12,
15]. In a study by Ahmad et al. [
18], 41 patients with type 2 diabetes with high serum urea levels and normal liver enzymes consumed 0.7 g of
N. sativa in the form of oil daily for 40 days. After measuring ALT and AST levels, they received a placebo again for 40 days, capsules of wheat bran were as placebo, then again liver enzyme levels of ALT and AST were measured. A significant increase in AST levels and an insignificant increase in ALT levels were seen in people consuming
N. sativa. Also, insulin level increased significantly after intervention. During the study, diet and dosage of medications for diabetes in participants were constant and usual [
18]. Another study in central obese men showed that taking two 750 mg
N. sativa capsules, 2 times, daily for three months reduced AST, ALT insignificantly but decreased body weight, waist circumference (WC), blood pressure and creatinine significantly. Intervention group had low HDL and high TG, WC and systolic blood pressure and normal ALT and AST. The placebo was capsules of 750 mg flour [
23].
Qidwai et al. [
22] also showed that supplementation with 2
N. sativa capsules at a dose of 500 mg twice daily for 6 weeks after meals had a non-significant reduction effect on ALT levels compared to placebo group, the control group took 2 capsules contained calcium lactate, twice daily. The basic total cholesterol in people was high. Only one study examined the effect of
N. sativa on the ALP enzyme level. In this study, Valizadeh et al. [
17], reported that supplementation of 12 postmenopausal women with osteoporosis with
N. sativa oil for 3 months at the dose of 3 mL/0.05 mL/kg/day was associated with a non-significant increase in serum ALP and AST levels and a non-significant decrease in bone ALP levels. However, a significant decrease in serum ALT levels compared to the placebo group was observed. All participants received calcium-D supplements (2 tablets per day) throughout the study for 3 months period.
Also, 2 studies have examined the effect of
N. sativa supplementation on γ-glutamyltrasnferase (GGT) level [
14,
16]. In 2017 in Pakistan, a study was conducted on NAFLD overweight patients with high ALT and AST in which people were divided into 2 categories of placebo and intervention. The number of people in each group was 35 and the intervention group after supplementation with
N. sativa capsule for 12 weeks at a dose of 1 g/2 times/day, were biochemically evaluated, then it was found that serum GGT level decreased insignificantly while serum ALT and AST and fatty liver grading reduced significantly [
14]. In another study on NAFLD overweight patients with high AST and fasting blood sugar (FBS) in
N. sativa group, after supplementation of 22 patients with one g of
N. sativa capsule daily for 8 weeks, liver enzymes (ALT, AST, and GGT), inflammatory markers (high sensitivity C-reactive protein [hs-CRP], tumor necrosis factor-α [TNF-α], and interleukin [IL]-6), insulin, lipid profiles (total cholesterol, TG, very low density lipoprotein, LDL-C, and HDL-C), FBS, and blood pressure were measured. Consumption of
N. sativa seed oil as supplement decreased liver enzymes (AST and ALT) and compared to the placebo group, however, had no significant effect on serum GGT level in comparison with the beginning of the study, also lipid profile and FBS in intervention group decreased significantly [
16].
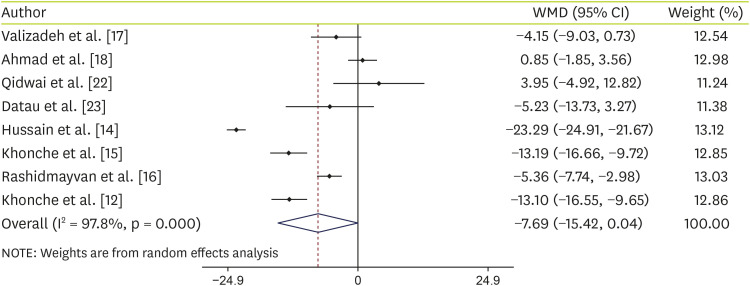
Effect of N. sativa supplementation on ALT
Data analysis of 8 studies on ALT shows that supplementation with
N. sativa non-significantly decreased ALT levels (WMD, −7.26 IU/L; 95% CI, −15.42, 0.04; p = 0.051) with significant heterogeneity (I-squared, 97.8%; p < 0.001) (
Figure 2). To find any source of heterogeneity, we performed subgroup analyses based on intervention type (capsules or oil), age (≤ 40 or > 40), trial duration (< 12 weeks or ≥ 12 weeks) and sample size (≤ 50 or > 50). Details of subgroup analyses are shown in
Table 3.
N. sativa supplementation showed a significant reduction in ALT levels at the sample size > 50 and the duration of the study ≥ 12 weeks.
Figure 2
Forest plot detailing WMD and 95% CIs for the effect of Nigella sativa on ALT.
WMD, weighted mean difference; CI, confidence interval; ALT, alanine aminotransferase.
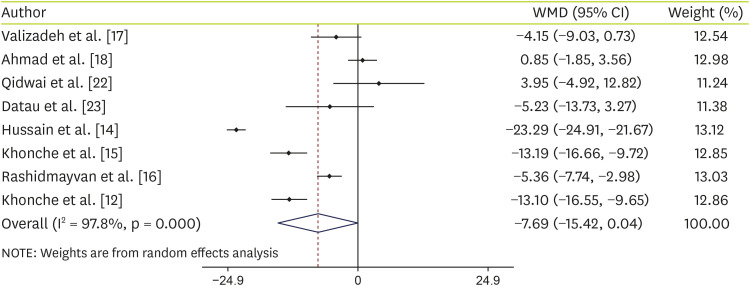
Table 3Subgroup analysis of included randomized controlled trials in meta-analysis of the effect of Nigella sativa supplementation on ALT and AST levels
Table 3
|
Group |
No. of effect size |
WMD (95% CI) |
p value |
I2 (%) |
p-heterogeneity |
p for between subgroup heterogeneity |
|
ALT |
|
|
|
|
|
|
|
Intervention |
|
|
|
|
|
< 0.001 |
|
|
Oil |
4 |
−7.39 (−15.07, 0.28) |
0.059 |
94.8 |
< 0.001 |
|
|
Capsule |
4 |
−7.87 (−20.73, 4.99) |
0.230 |
98.3 |
< 0.001 |
|
Mean age |
|
|
|
|
|
< 0.001 |
|
|
≤ 40 |
3 |
−11.52 (−25.83, 2.80) |
0.115 |
95.7 |
< 0.001 |
|
|
> 40 |
5 |
−5.53 (−12.58, 1.52) |
0.124 |
93.6 |
< 0.001 |
|
Duration (wk) |
|
|
|
|
|
< 0.001 |
|
|
< 12 |
3 |
−1.03 (−6.43, 4.36) |
0.707 |
85.1 |
< 0.001 |
|
|
≥ 12 |
5 |
−12.18 (−19.81, −4.55) |
0.002 |
95.8 |
< 0.001 |
|
Sample size |
|
|
|
|
|
< 0.001 |
|
|
≤ 50 |
5 |
−3.12 (−6.95, 0.71) |
0.110 |
75.1 |
< 0.001 |
|
|
> 50 |
3 |
−12.32 (−20.67, −3.96) |
0.004 |
95.9 |
< 0.001 |
|
AST |
|
|
|
|
|
|
|
Intervention |
|
|
|
|
|
< 0.001 |
|
|
Oil |
4 |
−12.79 (−18.97, −6.62) |
< 0.001 |
92.3 |
< 0.001 |
|
|
Capsule |
3 |
−4.62 (−12.00, 2.77) |
0.220 |
94.6 |
< 0.001 |
|
Mean age |
|
|
|
|
|
< 0.001 |
|
|
≤ 40 |
3 |
−12.79 (−18.97, −6.62) |
< 0.001 |
92.3 |
< 0.001 |
|
|
> 40 |
4 |
−4.62 (−12.00, 2.77) |
0.220 |
94.6 |
< 0.001 |
|
Duration (wk) |
|
|
|
|
|
< 0.001 |
|
|
< 12 |
2 |
−6.79 (−22.08, 8.50) |
0.384 |
97.5 |
< 0.001 |
|
|
≥ 12 |
5 |
−8.72 (−14.60, −2.83) |
0.004 |
95.0 |
< 0.001 |
|
Sample size |
|
|
|
|
|
< 0.001 |
|
|
≤ 50 |
4 |
−13.43 (−17.78, −9.08) |
0.340 |
94.3 |
< 0.001 |
|
|
> 50 |
3 |
−3.99 (−12.22, 4.23) |
< 0.001 |
89.6 |
< 0.001 |
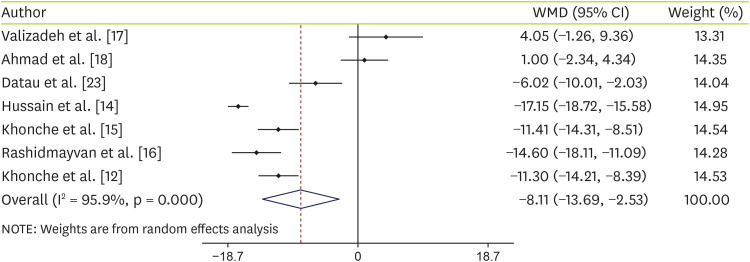
Effect of N. sativa supplementation on AST
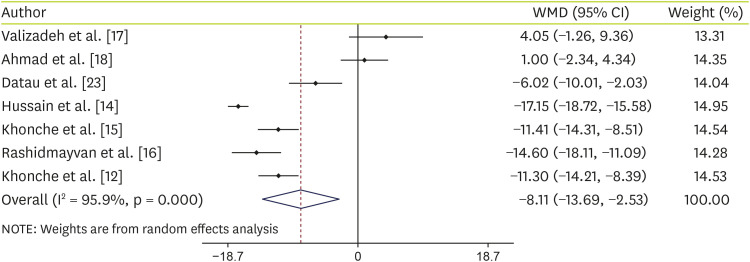
Data analysis of 7 studies on AST showed that supplementation with
N. sativa significantly decreased AST levels (WMD, −8.11 IU/L; 95% CI, −13.69, −2.53; p = 0.004) with significant heterogeneity (I-squared, 95.9%; p < 0.001) (
Figure 3). Details of subgroup analyses are shown in
Table 3. Analyses showed that if the age of the people is equal to or less than 40 years or people supplemented with
N. sativa oil or the duration of supplementation was ≥ 12 weeks,
N. sativa decrease AST levels significantly.
Figure 3
Forest plot detailing WMD and 95% CIs for the effect of Nigella sativa on AST.
WMD, weighted mean difference; CI, confidence interval; AST, aspartate aminotransferase.
DISCUSSION
To the best of our knowledge, this is the first meta‐analysis that examined the efficacy of N. sativa supplementation on liver enzymes. There were many studies on the effect of N. sativa supplementation on liver enzymes, but many of them have been on animal and in vitro studies. Also, some of the human studies did not have a placebo group, and some of them used a combination of N. sativa with other substances such as lemon balm. Finally, we examined 8 RCTs for ALT and 7 RCTs for AST. We found that supplementation with N. sativa may significantly reduce AST levels with a non-significant reduction effect on ALT.
In line with our findings, Khonche et al. [
12] showed that supplementation with 2.5 mL fully standardized
N. sativa seed oil every 12 hours for 3 months reduced significantly TG, LDL-C, AST, ALT levels and grade of hepatic steatosis, with an increase in HDL-C. Also with the same dose and duration of treatment,
N. sativa seed oil reduced significantly AST, ALT levels and liver status was significantly better compared to the control group by three-month ultrasound examination [
15]. Moreover, 1 g
N. sativa oil capsule daily for 8 weeks decreased the FBS level, lipid profiles, liver enzymes, hs-CRP, IL-6, and TNF-α, while it increased the HDL-C levels, compared to the placebo group and had no significant effect on serum levels of insulin, blood pressure, and GGT in comparison with the beginning of the study [
16]. In another study, supplementation with the 1 g
N. sativa oil capsule twice a day for 3 months decreased significantly body weight, body mass index, AST and ALT levels [
14]. These findings have been shown in people with NAFLD. While other evidence suggests that supplementation with
N. sativa has no significant effect on ALT and AST levels [
17,
18,
22,
23]. In a study by Valizadeh et al. [
17] in 12 postmenopausal women,
N. sativa oil for 3 months at the dose of 3 mL/0.05 mL/kg/day was associated with a non-significant increase in serum ALP and AST levels, a non-significant decrease in bone ALP levels and a significant decrease in serum ALT levels compared to the placebo group which shows a bone metabolism and the release of ALP from the bone into the blood due to osteoporosis should also be considered in this study. To explain the controversial results observed among studies, differences in sample size, type of intervention, age of participants and study duration should be taken into account. To explain the results of this meta-analysis, it can be said that the number of articles that measured the effect of supplementation of
N. sativa on AST level was less than ALT and the most of these studies had shown a significant effect on AST, also the reduction effect of this supplement on AST was higher than ALT in studies.
So far, no specific therapeutic agent has been found for the treatment or prevention of NAFLD. Accumulating evidence exists on the effect of complementary and alternative therapies [
16]. It has been shown that omega-3 fatty acid may help to treat NAFLD [
24]. Additionally, the effectiveness of vitamin E and pioglitazone have been shown in the treatment of NAFLD [
5]. However, there are concerns about the safety of pioglitazone and long-term vitamin E intake at high doses (400 units per day) for the treatment of NAFLD because vitamin E has been reported to increase the risk of all-cause mortality. Moreover, effective treatments for NAFLD should aim to reduce insulin resistance and metabolic risk factors [
25]. Pharmacological studies have shown that
N. sativa can be effective in treating NAFLD [
12]. One study found that oral
N. sativa significantly improved dyslipidemia, high blood TNF-α and malondialdehyde levels, and hepatic steatosis in the rat model with high-fructose diet-induced steatohepatitis [
26]. In addition,
N. sativa prevented liver damage in a variety of clinical laboratory studies [
27]. Since NAFLD is commonly associated with dyslipidemia [
1], TG and LDL-C-reducing effects and HDL-enhancing effects of
N. sativa oil can be useful for NAFLD patients [
28]. Additionally,
N. sativa reduces the body weight and fasting blood levels of total cholesterol, LDL-C, and glucose in diabetic and metabolic syndrome patients, thus it has direct and indirect improving effects on liver status of patients [
8]. Various mechanisms of the effect of
N. sativa on NAFLD have been reported including choleretic effect [
29], the interaction with α1-acid glycoprotein [
22], up-regulation of PPARγ [
16,
24,
25], anti-inflammatory, anti-apoptotic, anti-fibrotic and anti-oxidant activities [
26], inhibiting IRAK1 and subsequently nuclear factor-κB and activator protein-1 [
30].
Several limitations could be mentioned for the current meta-analysis: the most important one is related to the relatively small number of trials that met the specified inclusion criteria. Also, there was a significant heterogeneity among the studies, although the random effect model was used. In addition, due to the limited number of studies, only 2 liver enzymes were evaluated. Finally, most of the studies considered showed bias, and thus it is hard to reach a definitive conclusion. Given the limitations, the findings of the present review should be interpreted with caution, in addition, more studies are needed to found effects of N. sativa on liver enzymes in patients with NAFLD.
CONCLUSIONS
This meta-analysis showed that N. sativa supplementation significantly reduces AST levels and insignificantly ALT levels, however more RCTs are needed to found effects of N. sativa on liver enzymes in patients with NAFLD and levels of other liver enzymes should also be evaluated.
NOTES
-
Conflict of Interest: The authors declare that they have no competing interests.
SUPPLEMENTARY MATERIAL
REFERENCES
- 1. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med 2018;24:908-922.
- 2. Ashtari S, Pourhoseingholi MA, Zali MR. Non-alcohol fatty liver disease in Asia: prevention and planning. World J Hepatol 2015;7:1788-1796.
- 3. Aller R, Izaola O, Gómez S, Tafur C, González G, Berroa E, Mora N, González JM, de Luis DA. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur Rev Med Pharmacol Sci 2015;19:3118-3124.
- 4. Ji HF, Sun Y, Shen L. Effect of vitamin E supplementation on aminotransferase levels in patients with NAFLD, NASH, and CHC: results from a meta-analysis. Nutrition 2014;30:986-991.
- 5. Hung CK, Bodenheimer HC Jr. Current treatment of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin Liver Dis 2018;22:175-187.
- 6. Tavakkoli A, Ahmadi A, Razavi BM, Hosseinzadeh H. Black seed (Nigella sativa) and its constituent thymoquinone as an antidote or a protective agent against natural or chemical toxicities. Iran J Pharm Res 2017;16:2-23.
- 7. Kaatabi H, Bamosa AO, Lebda FM, Al Elq AH, Al-Sultan AI. Favorable impact of Nigella sativa seeds on lipid profile in type 2 diabetic patients. J Family Community Med 2012;19:155-161.
- 8. Fallah Huseini H, Amini M, Mohtashami R, Ghamarchehre ME, Sadeqhi Z, Kianbakht S, Fallah Huseini A. Blood pressure lowering effect of Nigella sativa L. seed oil in healthy volunteers: a randomized, double-blind, placebo-controlled clinical trial. Phytother Res 2013;27:1849-1853.
- 9. Heshmati J, Namazi N, Memarzadeh MR, Taghizadeh M, Kolahdooz F.
Nigella sativa oil affects glucose metabolism and lipid concentrations in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Food Res Int 2015;70:87-93.
- 10. Ibrahim RM, Hamdan NS, Ismail M, Saini SM, Abd Rashid SN, Abd Latiff L, Mahmud R. Protective effects of Nigella sativa on metabolic syndrome in menopausal women. Adv Pharm Bull 2014;4:29-33.
- 11. Tasawar Z, Siraj Z, Ahmad N, Lashari MH. The effects of Nigella sativa (Kalonji) on lipid profile in patients with stable coronary artery disease in Multan, Pakistan. Pak J Nutr 2011;10:162-167.
- 12. Khonche A, Huseini HF, Gholamian M, Mohtashami R, Nabati F, Kianbakht S. Standardized Nigella sativa seed oil ameliorates hepatic steatosis, aminotransferase and lipid levels in non-alcoholic fatty liver disease: a randomized, double-blind and placebo-controlled clinical trial. J Ethnopharmacol 2019;234:106-111.
- 13. Benhaddou-Andaloussi A, Martineau LC, Vallerand D, Haddad Y, Afshar A, Settaf A, Haddad PS. Multiple molecular targets underlie the antidiabetic effect of Nigella sativa seed extract in skeletal muscle, adipocyte and liver cells. Diabetes Obes Metab 2010;12:148-157.
- 14. Hussain M, Tunio AG, Akhtar L, Shaikh GS. Effects of Nigella sativa on various parameters in patients of non-alcoholic fatty liver disease. J Ayub Med Coll Abbottabad 2017;29:403-407.
- 15. Khonche A, Gholamian M, Kianbakht S, Husseini H, Mohtashami R, Bayatpoor ME, Mirzadeh M, Mashayekh A, Hosseini MA. Effectiveness of Nigella sativa oil on patients with non-alcoholic fatty liver: a randomized double-blind placebo-controlled trial. Acad J Med Plants 2018;6:307-312.
- 16. Rashidmayvan M, Mohammadshahi M, Seyedian SS, Haghighizadeh MH. The effect of Nigella sativa oil on serum levels of inflammatory markers, liver enzymes, lipid profile, insulin and fasting blood sugar in patients with non-alcoholic fatty liver. J Diabetes Metab Disord 2019;18:453-459.
- 17. Valizadeh N, Zakeri HR, Amin Asnafi G, Shafiee A, Sarkhail P, Heshmat R, Sereshti H, Larijani B. Impact of Black seed (Nigella sativa) extract on bone turnover markers in postmenopausal women with osteoporosis. DARU J Pharm Sci 2009;17:20-25.
- 18. Ahmad B, Masud T, Uppal AM, Naveed AK. Effects of Nigella sativa oil on some blood parameters in type 2 diabetes mellitus patients. Asian J Chem 2009;21:5373-5381.
- 19. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. Cochrane Bias Methods Group. Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.
- 20. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13.
- 21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-188.
- 22. Qidwai W, Hamza HB, Qureshi R, Gilani A. Effectiveness, safety, and tolerability of powdered Nigella sativa (kalonji) seed in capsules on serum lipid levels, blood sugar, blood pressure, and body weight in adults: results of a randomized, double-blind controlled trial. J Altern Complement Med 2009;15:639-644.
- 23. Datau EA, Wardhana , Surachmanto EE, Pandelaki K, Langi JA, Fias . Efficacy of Nigella sativa on serum free testosterone and metabolic disturbances in central obese male. Acta Med Indones 2010;42:130-134.
- 24. Schwenger KJ, Allard JP. Clinical approaches to non-alcoholic fatty liver disease. World J Gastroenterol 2014;20:1712-1723.
- 25. Meral I, Yener Z, Kahraman T, Mert N. Effect of Nigella sativa on glucose concentration, lipid peroxidation, anti-oxidant defence system and liver damage in experimentally-induced diabetic rabbits. J Vet Med A Physiol Pathol Clin Med 2001;48:593-599.
- 26. Al-Okbi SY. Role of nutraceuticals in prevention of non-alcoholic fatty liver. Plant- and marine-based phytochemicals for human health. Palm Bay: Apple Academic Press; 2018.
- 27. Mollazadeh H, Hosseinzadeh H. The protective effect of Nigella sativa against liver injury: a review. Iran J Basic Med Sci 2014;17:958-966.
- 28. Zaoui A, Cherrah Y, Mahassini N, Alaoui K, Amarouch H, Hassar M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine 2002;9:69-74.
- 29. Mollazadeh H, Afshari AR, Hosseinzadeh H. Review on the potential therapeutic roles of Nigella sativa in the treatment of patients with cancer: involvement of apoptosis: - black cumin and cancer. J Pharmacopuncture 2017;20:158-172.
- 30. Hossen MJ, Yang WS, Kim D, Aravinthan A, Kim JH, Cho JY. Thymoquinone: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci Rep 2017;7:42995.