ABSTRACT
This systematic review and meta-analysis of randomized controlled trials (RCTs) aimed to test our hypothesis that herbal tea may improve anthropometric parameters, metabolic factors, and hormone levels in women with polycystic ovarian syndrome (PCOS). A literature search was conducted on Information Sciences Institute, Medline (PubMed), Scopus, Embase, and Google Scholar, up to March 2023 without applying language or date restrictions. RCTs that assigned herbal tea vs. placebo on PCOS women and evaluated changes in anthropometric measurements, metabolic indices, or hormonal profiles were included. Six RCTs with 235 PCOS women (119 in the intervention and 116 in the control group) were included. Meta-analysis showed that herbal tea consumption led to significant decreases in weight (weighted mean difference [WMD], −2.02 kg; 95% confidence interval [CI], −3.25, −0.80), body mass index (BMI) (WMD, −0.88 kg/m2; 95% CI, −1.47, −0.28) and fasting blood glucose (FBG) (WMD, −6.47 mg/dL; 95% CI, −8.49, −4.45), compared to the control group. Herbal tea supplementation has also significantly increased follicle-stimulating hormone (FSH) concentration (WMD, 0.56 IU/L; 95% CI, 0.17, 0.95). Meanwhile, the effect of herbal tea on the waist/hip ratio, hip circumference, waist circumference, body fat, fasting insulin, FBG/insulin ratio, luteinizing hormone, total testosterone, and dehydroepiandrosterone sulfate was not significant. Herbal tea might be a potential supplemental therapy to manage weight, BMI, FBG, and FSH in PCOS women. Further large randomized clinical trials are recommended to affirm these findings.
-
Keywords: Herbal tea; Polycystic ovarian syndrome; Metabolic indicators; Systematic review; Meta-analysis
INTRODUCTION
Polycystic ovarian syndrome (PCOS), a widespread endocrine disorder in women, could result in non-ovulation infertility. About 7 percent of women of reproductive age suffer from this syndrome [
1]. Besides infertility, PCOS could lead to clinical complications such as amenorrhea or oligomenorrhea, hirsutism, acne, baldness, endometriosis, and premature menopause as well as metabolic disorders including insulin resistance (IR), type 2 diabetes (T2DM), and lipid profile disorders [
2,
3,
4]. Moreover, women with PCOS have a higher risk of cardiovascular diseases [
5,
6], also about 40%–50% of them are overweight or obese [
7,
8]. Treatment of PCOS complications such as infertility, menstruation disorders, and diabetes poses a lot of economic burden on the health care systems of countries; so, finding an effective remedy to cure this syndrome can reduce economic costs and improve health consequences [
9].
Several factors including genetics, environment, and lifestyle play roles in the etiology of PCOS [
10]. Lifestyle changes, including weight reduction and switching to a healthy diet, might be effective in treating PCOS [
11]; but following weight loss diets for a long time and maintaining reduced weight are difficult for these patients. On the other hand, due to the adverse effects of chemical and industrial drugs, alternative plant-based complementary medicine is becoming more and more popular [
12]. One of these complementary therapies includes anti-androgen components [
13], which are found in some herbal teas as daily beverages [
14,
15,
16].
Several previous researches have evaluated the effects of various herbal teas such as green tea, spearmint, and marjoram tea on females with PCOS. Although some clinical trials have found positive effects of herbal tea on anthropometric measurements [
17,
18], others did not confirm these findings [
14]. These inconsistencies have also been shown in the case of the impact of herbal tea on hormone levels and lipid profiles in PCOS [
16,
18,
19]. Therefore, to test our hypothesis that herbal tea may improve anthropometric parameters, metabolic factors, and hormone levels in women with PCOS, we conducted a systematic review and if possible, a meta-analysis of all published randomized controlled trials (RCTs) that investigated the effects of herbal tea on these parameters in women with PCOS.
MATERIALS AND METHODS
Search strategy
We collected data from the Information Sciences Institute, Medline/PubMed, Scopus, Embase electronic databases, and Google Scholar. Information was retrieved from the databases until March 2023, without any restriction in time or language. The following MeSH and non-MeSH terms were applied in the search strategy: Tea AND (PCOS OR "polycystic ovarian syndrome" OR "Polycystic Ovary Syndrome") AND ("Intervention Studies" OR intervention OR "controlled trial" OR randomized OR random OR trial OR randomly OR placebo OR blind OR blinded OR assign*). In addition, the references of related articles and reviews were also screened to find any other relevant trials. This research was reported based on Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines and the study protocol was also registered in PROSPRO (No: CRD42020212755).
Inclusion criteria
Investigations were eligible if they: 1) were RCTs with parallel or cross-over design; 2) assigned herbal tea vs. placebo to PCOS women; 3) reported changes or mean values and standard deviation (SD) for one or more main outcomes of interest, including anthropometric measurements (weight, body mass index [BMI], waist/hip ratio [WHR], percent of body fat content), lipid profile (high-density lipoprotein [HDL], low-density lipoprotein [LDL], triglyceride and total cholesterol [TC]), glycemic indices (fasting blood glucose [FBG], fasting insulin, homeostatic model assessment for IR [HOMA-IR]) or hormonal profile (luteinizing hormone [LH], follicle-stimulating hormone [FSH], testosterone).
Exclusion criteria
The following exclusion criteria were used: 1) trials without a control group; 2) trials without sufficient information; 3) animal and laboratory studies; 4) reviews, conference abstracts, commentaries, and case reports; 5) duplicate publications from the same trials; 6) RCTs that included the women with hirsutism and did not consider other PCOS criteria to define the disorder.
Data extraction
Two authors (E.A. and Z.H.) independently extracted the required information, including the name of the first author, publication year, characteristics of trial participants (such as number of participants in intervention and control group, mean age, and age range), duration of the intervention in week, design of the trial, definition of the intervention (content and dose of tea) and related control, means and SDs of outcomes of interest in included studies (anthropometric measurements, biochemical profile or hormonal profile) before and after supplementation.
Quality assessment
Cochrane quality assessment tool was used to assess the quality of each included study [
20]. According to this tool, each trial was assessed based on the following study characteristics: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias) selective reporting (reporting bias) and other sources of bias (detection bias). We independently categorized each RCT as low (L), high (H), and unclear (U) risk of bias for each domain. Finally, the overall quality of the study was categorized into weak, fair, or good, if < 3, 3, or ≥ 4 domains were respectively rated as low risk.
To compare two groups of herbal tea intake and control, means (± SDs) of differences in anthropometric measurements, lipid profile, glycemic indices, and hormonal profile were used to calculate the overall effect size. Statistical heterogeneity between studies was calculated by Cochran’s Q test and I
2. Values for I
2 values of 0%–25%, 25%–50%, 50%–75%, and 75%–100% are respectively referred to as low, moderate, substantial, and considerable heterogeneity. Sources of heterogeneity were found by performing subgroup analyses based on duration of intervention and intervention type. A fixed-effect model was used to evaluate subgroup heterogeneity. Furthermore, meta-regression and dose-response analysis were conducted to find sources of heterogeneity. The influence of a single study on the overall was explored by running a sensitivity analysis by eliminating one study and repeating the analysis. We assessed publication bias by visual inspection of asymmetry in funnel plots, Begg’s test, and Egger’s regression test [
21]. Stata version 14 (StataCorp.; College Station, TX, USA) was used for data analysis, and p < 0.05 was considered as statistically significant.
RESULTS
Study characteristics
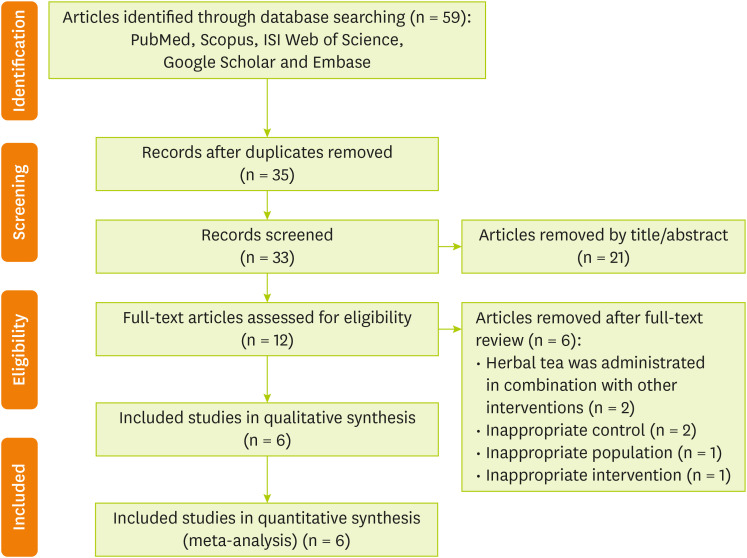
Figure 1 shows the flow diagram of the selection procedure. In brief, 59 papers were identified through database searching; 35 records remained after duplications were removed. By screening, 33 citations were retrieved that 21 of them were removed by skimming the title and abstracts. Full text of remained 12 studies were assessed for eligibility. After further examination of remained trials, 6 articles were excluded based on the following reasons: herbal tea was administrated in combination with other substances (n = 2), inappropriate control groups (n = 2), inappropriate population (n = 1), and inappropriate intervention (n = 1). Finally, six studies [
14,
15,
16,
17,
18,
19] were potentially eligible and included in the systematic review and meta-analysis.
Figure 1Flowchart describing the process of study selection.
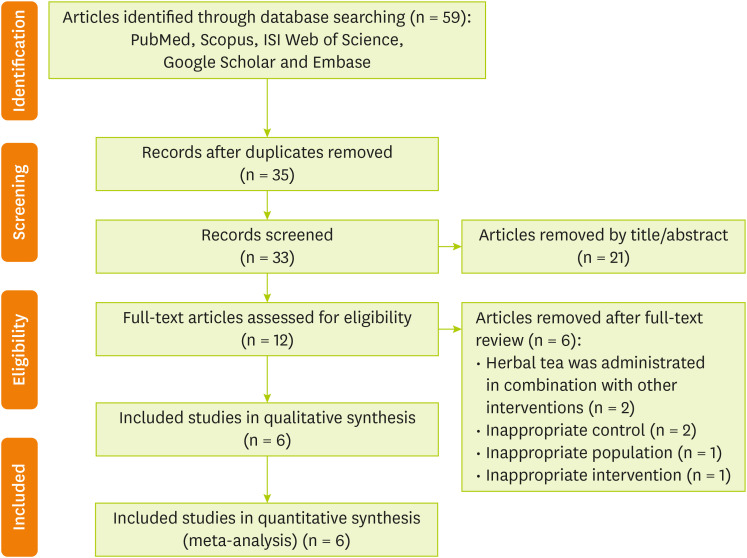
General characteristics of the included studies are shown in
Table 1. All eligible interventions had a parallel design and a total of 235 PCOS women (119 intervention and 116 control group) were included in the analysis. The mean age of participants in these studies ranged from 18 to 42 years and intervention duration of the studies ranged from 4 to 12 weeks. PCOS was defined based on Rotterdam criteria in all eligible RCTs. Included studies were published between 2006 and 2020, and were from Iran (n = 3) [
15,
17,
18], England (n = 1) [
19], China (n = 1) [
14], and Jordan (n = 1) [
16]. Different types of herbal tea including green tea (n = 4) [
14,
15,
17,
18], Spearmint tea (n = 1) [
19], and Marjoram tea (n = 1) [
16] were used as the intervention. The intervention dose of herbal tea was varied based on the type of intervention. chamomile tea, origanum syriacum L, corn starch, wheat flour, or an unknown placebo were used in control groups. Major outcomes that were identified from the studies included anthropometric measurements (weight, BMI, WHR, triceps skin fold, body fat content, waist and hip circumference [WC and HC]), biochemical profile (fasting insulin, FBG, FBG/insulin ratio, TC, triglyceride, LDL cholesterol, HDL cholesterol, HOMA-IR), hormonal profile (total testosterone, sex hormone-binding globulin, free androgen index, androstenedione, dehydroepiandrosterone sulfate [DHEAS], FSH, LH, E2, progesterone). The overall quality of included studies according to the domains used by the Cochrane Collaborations tool is provided in
Table 2 and
Supplementary Figure 1. All of the 6 studies included in the systematic review were categorized as good quality [
14,
15,
16,
17,
18,
19].
Table 1The main characteristics of the included studies examined the effect of herbal tea supplementation on women with polycystic ovarian syndrome
Table 1
|
Studies |
Country |
Mean age/age range |
No. of participants (intervention/control) |
Design |
Study duration (wk) |
Control |
Intervention (content/dose) |
Outcome |
Before intervention |
After intervention |
Overall quality |
|
Int. |
Con. |
Int. |
Con. |
|
Chan et al. [14] (2006) |
China |
25–40, 34.8 ± 4.2 |
34 (18/16) |
Parallel |
12 (3 mon) |
Placebo |
Green tea (2% Lung Chen tea freeze-dried powder, 6 capsules/day) |
Anthropometric measurements |
Weight (kg) |
75.72 ± 2.96 |
75.27 ± 3.29 |
74.72 ± 3.03 |
76.30 ± 3.67 |
Good |
|
BMI (kg/m2) |
30.75 ± 0.77 |
30.22 ± 0.95 |
30.40 ± 0.89 |
30.80 ± 0.84 |
|
WHR |
0.84 ± 0.20 |
0.85 ± 0.20 |
0.84 ± 0.20 |
0.87 ± 0.20 |
|
Body fat content (%) |
44.17 ± 1.87 |
41.85 ± 2.83 |
43.77 ± 1.89 |
43.47 ± 2.34 |
|
Biochemical profile |
Fasting insulin (pmol/L) |
89.03 ± 29.34 |
134.03 ± 76.32 |
52.78 ± 21.60 |
92.01 ± 60.21 |
|
FBG (mg/dL) |
91.80 ± 4.14 |
96.30 ± 7.20 |
91.80 ± 4.68 |
103.50 ± 4.50 |
|
FBG/I ratio (mmol/mIU) |
0.88 ± 0.49 |
0.43 ± 0.28 |
0.61 ± 0.24 |
0.93 ± 0.75 |
|
Fasting cholesterol (mM) |
4.67 ± 0.53 |
5.10 ± 0.35 |
0.87 ± 0.48 |
5.20 ± 0.45 |
|
Fasting triglyceride (mM) |
1.32 ± 0.38 |
1.54 ± 0.42 |
1.24 ± 0.28 |
1.60 ± 0.42 |
|
Fasting LDL-C (mM) |
2.84 ± 0.42 |
3.35 ± 0.39 |
3.06 ± 0.39 |
3.30 ± 0.36 |
|
Fasting HDL-C (mM) |
1.02 ± 0.22 |
1.05 ± 0.21 |
1.08 ± 0.22 |
1.08 ± 0.21 |
|
Fasting non-HDL-C (mM) |
3.70 ± 0.56 |
4.06 ± 0.36 |
3.71 ± 0.52 |
4.18 ± 0.38 |
|
Hormonal profile |
Total testosterone (ng/mL) |
0.22 ± 0.07 |
0.26 ± 0.07 |
0.23 ± 0.06 |
0.24 ± 0.09 |
|
SHBG (nM) |
133.00 ± 19.06 |
104.25 ± 13.59 |
134.00 ± 12.69 |
99.75 ± 17.61 |
|
Free androgen index |
0.76 ± 0.29 |
0.90 ± 0.36 |
0.71 ± 0.26 |
0.85 ± 0.33 |
|
Androstenedione (ng/mL) |
2.90 ± 0.50 |
3.40 ± 0.45 |
2.55 ± 0.50 |
3.27 ± 0.87 |
|
DHEAS (µg/mL) |
1.15 ± 0.30 |
1.30 ± 0.38 |
1.32 ± 0.41 |
1.41 ± 0.43 |
|
FSH (IU/L) |
5.37 ± 0.37 |
4.87 ± 0.48 |
5.35 ± 0.50 |
4.42 ± 0.42 |
|
LH (IU/L) |
4.50 ± 1.29 |
4.25 ± 1.11 |
3.95 ± 0.95 |
4.40 ± 1.61 |
|
Grant [19] (2010) |
Eastbourne, England |
19–42, 25.5 |
41 (20/21) |
Parallel |
4 (30 day) |
Chamomile |
Spearmint herbal tea (standardized content of dried tea, 2 capsules/day) |
Hormonal profiles |
Free testosterone (pg/mL) |
5.12 ± 2.14 |
4.98 ± 2.84 |
3.64 ± 2.67 |
4.49 ± 1.67 |
Good |
|
Total testosterone (ng/mL) |
0.81 ± 0.39 |
0.87 ± 0.40 |
0.62 ± 0.34 |
0.80 ± 0.14 |
|
DHEAS (µg/mL) |
184.50 ± 82.10 |
179.50 ± 85.30 |
183.30 ± 87.80 |
183.30 ± 82.80 |
|
FSH (IU/L) |
5.12 ± 1.98 |
5.67 ± 1.99 |
6.10 ± 2.10 |
5.59 ± 2.40 |
|
LH (IU/L) |
5.25 ± 3.20 |
5.47 ± 2.70 |
7.23 ± 3.90 |
5.23 ± 2.80 |
|
Haj-Husein et al. [16] (2016) |
Amman, Jordan |
21 ± 1.3 |
25 (14/11) |
Parallel |
4 (1 mon) |
Origanum syriacum L. |
Marjoram tea (0.2 g dried tea, 2 cups/day) |
Anthropometric measurements |
Weight (kg) |
66.04 ± 10.62 |
67.02 ± 9.52 |
65.80 ± 10.85 |
66.73 ± 9.52 |
Good |
|
Biochemical profile |
Fasting insulin (pmol/L) |
52.78 ± 19.44 |
53.75 ± 37.08 |
39.65 ± 15.28 |
54.86 ± 32.43 |
|
FBG (mg/dL) |
79.93 ± 14.07 |
81.60 ± 8.52 |
79.21 ± 10.96 |
85.90 ± 11.27 |
|
FBG/I ratio |
11.62 (3.85 |
17.10 ± 10.18 |
13.40 ± 7.14 |
13.02 ± 4.80 |
|
HOMA-IR |
1.53 ± 0.67 |
1.50 ± 0.83 |
1.14 ± 0.52 |
1.68 ± 0.93 |
|
Hormonal profile |
Total testosterone (ng/mL) |
0.77 ± 0.52 |
0.73 ± 0.26 |
0.79 ± 0.52 |
0.73 ± 0.36 |
|
DHEAS (µg/mL) |
3.37 ± 2.04 |
3.00 ± 1.02 |
2.85 ± 1.61 |
3.02 ± 1.11 |
|
FSH (IU/L) |
4.43 ± 1.83 |
5.21 ± 1.89 |
4.80 ± 1.68 |
5.36 ± 2.02 |
|
LH (IU/L) |
7.17 ± 6.28 |
8.77 ± 10.87 |
7.42 ± 6.02 |
12.23 ± 10.74 |
|
E2 (pg/mL) |
47.00 ± 38.01 |
45.56 ± 26.26 |
37.92 ± 16.12 |
45.78 ± 23.78 |
|
Progesterone (ng/mL) |
2.55 ± 5.57 |
1.12 ± 1.92 |
0.41 ± 0.30 |
0.43 ± 0.23 |
|
Mombaini et al. [15] (2017) |
Ahvaz, Iran |
23.7 ± 6 |
45 (22/23) |
Parallel |
6.5 (45 day) |
Corn starch |
Green tea (500 mg C. sinensis L., 4 tablets/day) |
Anthropometric measurements |
Weight (kg) |
73.99 ± 21.53 |
71.40 ± 12.27 |
72.81 ± 21.1 |
70.55 ± 12.43 |
Good |
|
BMI (kg/m2) |
28.96 ± 6.93 |
28.90 ± 4.30 |
28.49 ± 6.73 |
28.55 ± 4.36 |
|
WHR |
0.79 ± 0.06 |
0.79 ± 0.05 |
0.75 ± 0.17 |
0.79 ± 0.06 |
|
Body fat (%) |
34.69 ± 8.74 |
34.84 ± 5.17 |
34.13 ± 8.45 |
34.42 ± 5.27 |
|
Waist (cm) |
86.72 ± 17.18 |
85.08 ± 8.89 |
85.27 ± 15.78 |
83.56 ± 9.16 |
|
Hip (cm) |
108.81 ± 14.65 |
106.73 ± 11.29 |
108.04 ± 13.83 |
106.08 ± 11.87 |
|
Tehraniet al. [18] (2017) |
Isfahan, Iran |
20–40 |
60 (30/30) |
Parallel |
12 |
Wheat flour |
Green tea (500 mg, 2 capsules/day) |
Anthropometric measurements |
Weight (kg) |
86.68 ± 6.86 |
86.28 ± 6.03 |
82.90 ± 6.09 |
86.37 ± 6.03 |
Good |
|
Biochemical profile |
Fasting insulin (pmol/L) |
86.68 ± 6.86 |
86.28 ± 6.09 |
82.90 ± 6.09 |
86.37 ± 6.03 |
|
Hormonal profile |
Free testosterone |
86.68 ± 6.86 |
86.28 ± 6.09 |
82.90 ± 6.09 |
86.37 ± 6.03 |
|
Farhadian et al. [17] (2020) |
Hamadan, Iran |
18–35 |
30 (15/15) |
Parallel |
12 |
Placebo |
Green tea (500 mg, 3 tablets/day) |
Anthropometrics measurements |
Weight (kg) |
75.33 ± 6.47 |
74.46 ± 7.59 |
72.81 ± 5.96 |
74.84 ± 8.19 |
Good |
|
BMI (kg/m2) |
29.32 ± 4.31 |
28.45 ± 2.64 |
28.02 ± 3.49 |
28.69 ± 2.90 |
|
WHR |
0.84 ± 0.04 |
0.81 ± 0.04 |
0.82 ± 0.05 |
0.81 ± 0.05 |
|
Waist (cm) |
88.86 ± 7.60 |
86.40 ± 7.78 |
85.60 ± 7.13 |
87.60 ± 8.87 |
|
Hip (cm) |
106.27 ± 9.07 |
106.67 ± 6.48 |
103.87 ± 7.38 |
107.53 ± 7.22 |
Table 2Quality assessment of included studies assessed the effects of herbal tea supplementation on women with polycystic ovarian syndrome
Table 2
|
Studies |
Sequence generation |
Allocation concealment |
Blinding of participants and personnel |
Blinding of outcome assessment |
Incomplete outcome data |
Selective Outcome reporting |
Other potential threats to validity |
Overall quality |
|
Chan et al. [14] (2006) |
L |
L |
L |
L |
L |
L |
U |
Good |
|
Grant [19] (2010) |
L |
L |
U |
L |
L |
L |
U |
Good |
|
Haj-Husein et al. [16] (2016) |
L |
L |
L |
L |
L |
L |
U |
Good |
|
Mombaini et al. [15] (2017) |
L |
U |
L |
L |
L |
L |
U |
Good |
|
Tehrani et al. [18] (2017) |
L |
L |
L |
U |
L |
L |
U |
Good |
|
Farhadian et al. [17] (2020) |
L |
L |
L |
U |
L |
L |
U |
Good |
Meta-analysis of the effect of herbal tea on anthropometric measurements in PCOS women
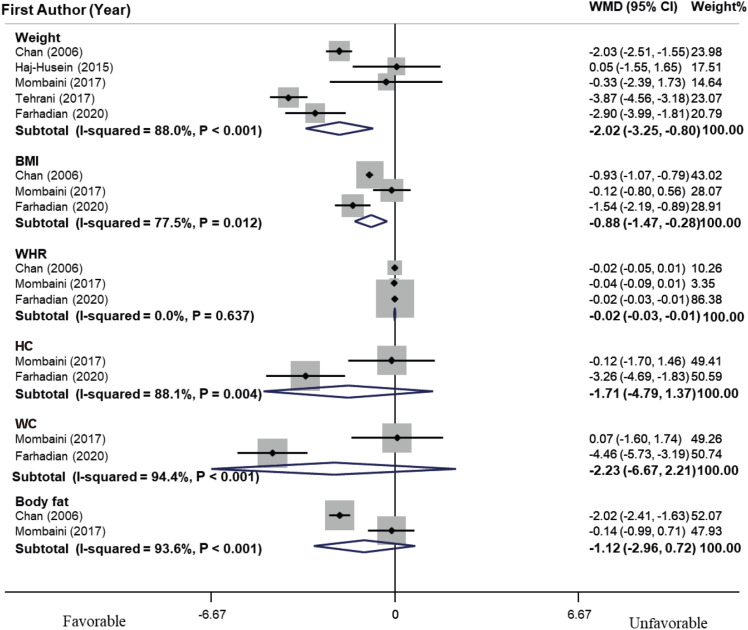
As shown in
Figure 2, the meta-analysis revealed a significant decrease in weight (weighted mean difference [WMD], −2.02 kg; 95% CI, −3.25, −0.80) and BMI (WMD, −0.88 kg/m
2; 95% CI, −1.47, −0.28) compared with the control group. Heterogeneity was considerable for the weight (I
2 = 88.0%, p < 0.001); so, subgroup analysis was conducted based on the type of intervention and duration of the intervention (< 6 vs. ≥ 6 weeks) (
Table 3). These subgroup analyses could not completely explain the source of heterogeneity. Hence, meta-regression analyses were conducted based on the mean age of participants and intervention duration (as continuous variables); none of these covariates could explain the source of heterogeneity (mean age of participants [β = −0.03, p = 0.69, I
2
residual = 96.7%] and intervention duration [β = −0.13, p = 0.23, I
2
residual = 96.79%]). Furthermore, dose-response analysis was performed to assess the effect of tea on weight and revealed that there was no linear relationship between the duration of herbal tea intervention and weight (p = 0.21). Sensitivity analysis determined that the overall effect would not significantly change by excluding each study. Publication bias analysis for weight manifested no significant publication bias (p = 0.62, Begg’s test, and p = 0.58, Egger’s test). Meanwhile, tea consumption did not have a significant effect on WHR, HC, WC, and body fat.
Figure 2
Forest plot of effects of herbal tea supplementation on anthropometric indices in women with polycystic ovarian syndrome. Meta-analysis revealed a significant decrease in weight (WMD, −2.02 kg; 95% CI, −3.25, −0.80) and BMI (WMD, −0.88 kg/m2; 95% CI, −1.47, −0.28) compared with the control group. Heterogeneity was considerable for weight (I2 = 88.0%, p < 0.001). Meanwhile, tea consumption did not have significant effect on WHR, HC, WC and body fat.
WMD, weighted mean difference; CI, confidence interval; BMI, body mass index; WHR, waist/hip ratio; HC, hip circumference; WC, waist circumference.
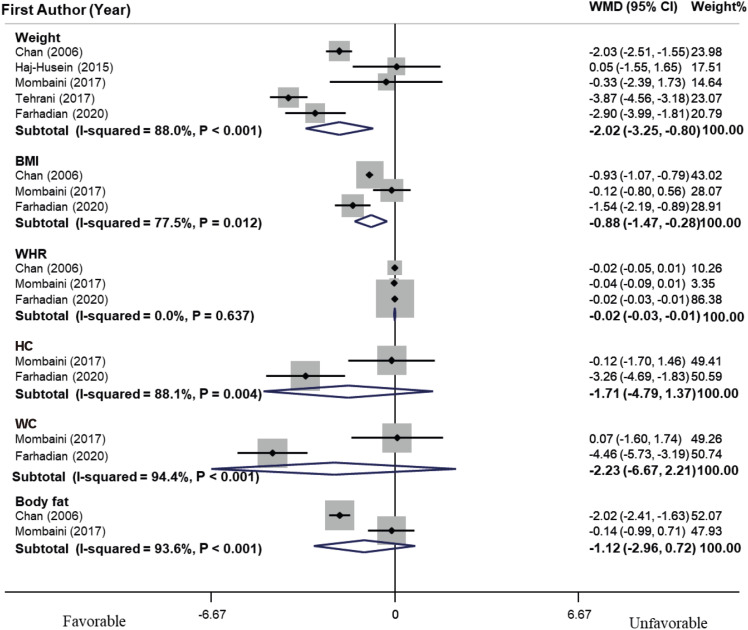
Table 3Results of subgroup-analysis for effect of herbal tea supplementation on weight in women with polycystic ovarian syndrome
Table 3
|
Subgroups |
No. of effect sizes |
WMD (95% CI) |
p within*
|
I2 (%) |
p between†
|
|
Overall |
5 |
−2.02 (−3.25, −0.8) |
< 0.001 |
88.00 |
|
|
Intervention |
|
|
|
|
0.002 |
|
Green tea |
4 |
−2.49 (−3.71, −1.27) |
< 0.001 |
87.2 |
|
Marjoram tea |
1 |
0.05 (−1.55, 1.65) |
- |
- |
|
Duration of intervention (wk) |
|
|
|
|
0.002 |
|
< 6 |
1 |
0.05 (−1.55, 1.65) |
- |
- |
|
≥ 6 |
4 |
−2.49 (−3.71, −1.27) |
< 0.001 |
87.2 |
Meta-analysis of the effect of herbal tea on the metabolic profile of PCOS women
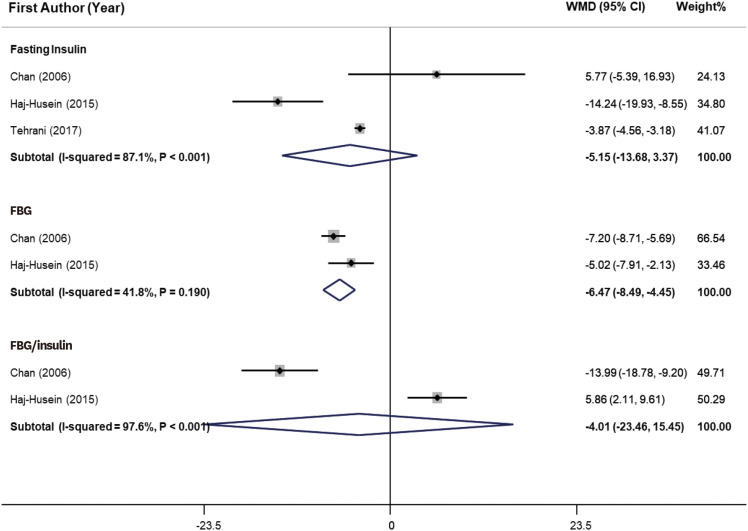
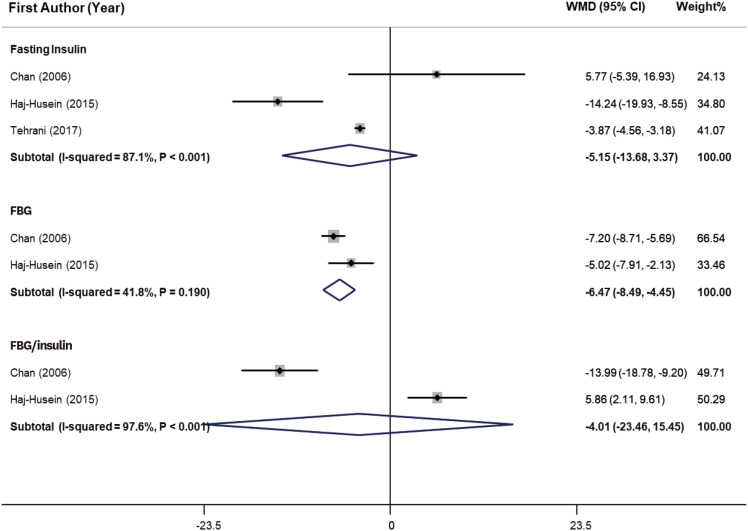
As shown in
Figure 3, herbal tea consumption significantly decreased FBG (WMD, −6.47 mg/dL; 95% CI, −8.49, −4.45) in PCOS women. Heterogeneity was not significant (I
2 = 41.8%, p = 0.19). However, tea consumption did not show a significant effect on fasting insulin and glucose/insulin ratio. We could not perform a meta-analysis for the effect of tea drinking on lipid profile, due to the few numbers of effect sizes.
Figure 3
Forest plot of effects of herbal tea supplementation on glycemic parameters in women with PCOS. Herbal tea consumption significantly decreased FBG (WMD, −6.47 mg/dL; 95% CI, −8.49, −4.45) in PCOS women. Heterogeneity was not significant (I2 = 41.8%, p = 0.19). However, tea consumption did not show significant effect on fasting insulin and FBG/insulin ratio.
WMD, weighted mean difference; CI, confidence interval; PCOS, polycystic ovarian syndrome; FBG, fasting blood glucose.
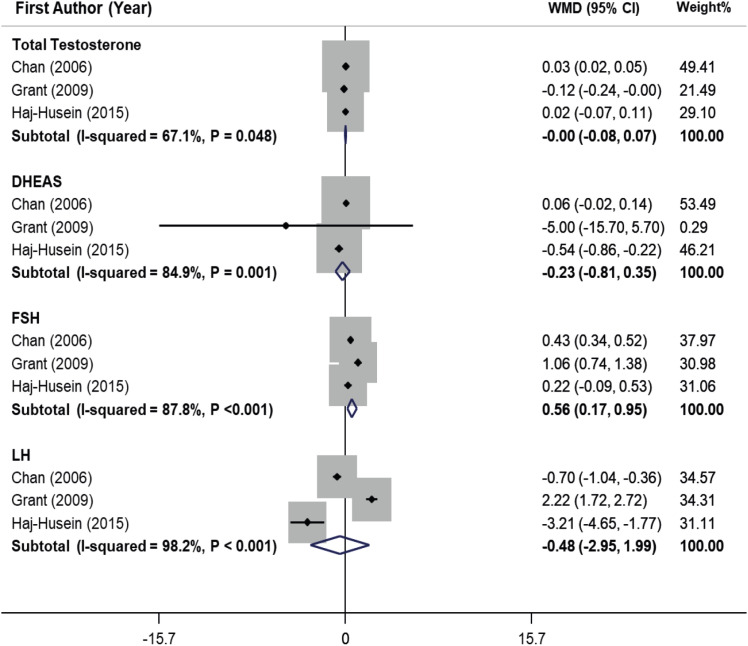
Meta-analysis of the effect of herbal tea on the hormonal profile of PCOS women
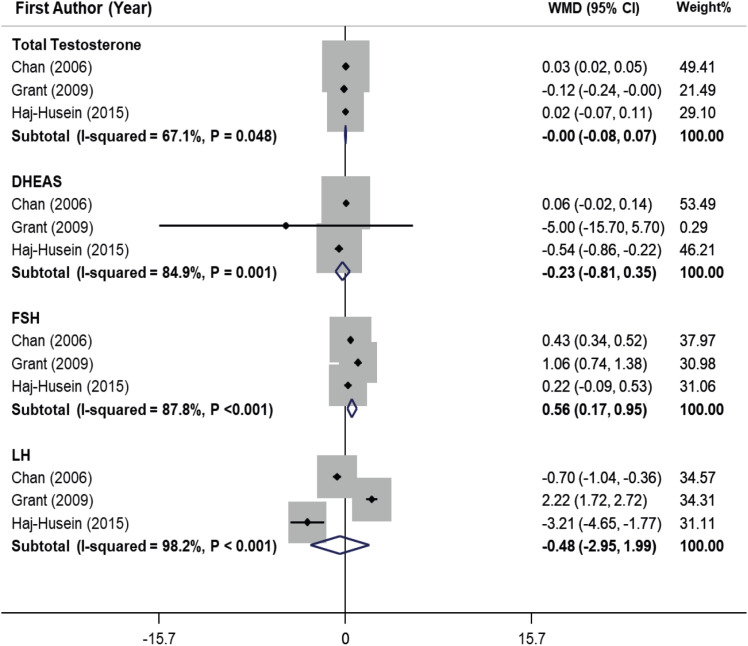
The pooling estimate of included studies declared that herbal tea consumption significantly increased FSH (WMD, 0.56 IU/L; 95% CI, 0.17, 0.95) in PCOS women (
Figure 4). Heterogeneity was considerable (I
2 = 87.8%, p < 0.001). On the other hand, tea consumption had no significant effect on total testosterone, DHEAS, and LH in women with PCOS.
Figure 4
Forest plot of effects of herbal tea supplementation on hormonal profile in women with PCOS. Pooling estimate of included studies declared that herbal tea consumption significantly increased follicle-stimulating hormone (WMD, 0.56 IU/L; 95% CI, 0.17, 0.95) in PCOS women. Heterogeneity was considerable (I2 = 87.8%, p < 0.001). On the other hand, tea consumption had no significant effect on total testosterone, DHEAS and luteinizing hormone in women with PCOS.
WMD, weighted mean difference; CI, confidence interval; DHEAS, dehydroepiandrosterone sulfate; FSH, follicle-stimulating hormone; LH, luteinizing hormone; PCOS, polycystic ovarian syndrome.
DISCUSSION
This meta-analysis on RCTs verified our hypothesis that herbal tea consumption might result in significant decreases in weight, BMI, and FBG and a significant increase in FSH in women with PCOS. In contrast to our hypothesis, tea consumption did not have a significant impact on WHR, HC, WC, body fat, fasting insulin, FBG/insulin ratio, total testosterone, DHEAS, and LH, maybe due to the few numbers of total eligible studies and small effect sizes. However, this is not the first systematic review and meta-analysis of RCTs that assessed the effect of herbal tea supplementation on metabolic profile, anthropometric indices, and hormonal status of women with PCOS. The main difference between our study and the previous one [
22] is that we evaluated some more parameters, and although they showed that consumption of tea supplementation in women with PCOS significantly decreased the levels of FBG, fasting insulin, and weight but they did not find a significant effect for BMI and FSH.
The main goals of PCOS therapy are modifying hyperandrogenic features, controlling metabolic abnormalities, and improving ovulation and fertility [
23]. Finding an herbal remedy to improve the health status of PCOS women, especially to reduce weight and modify the hormonal profiles and glycemic parameters, is so valuable. Emerging studies on phytomedicine and alternative plant-based medicine have shown promising results in the treatment of PCOS. One of these remedies is an herbal tea that has anti-androgen properties, which could reduce the levels or activity of androgen hormones within the human body [
13]. According to our study herbal teas such as spearmint tea, green tea, and marjoram tea may have these effects and cause all of the above clinical functions so some hormonal-related symptoms like amenorrhea or oligomenorrhea, hirsutism, and acne in women with PCOS would improve by increasing the levels of FSH.
In a recent systematic review and meta-analysis, Payab et al. [
24] found that intake of green tea in overweight and obese adults or those with metabolic syndrome led to significant reductions in weight, BMI, WC, HC, and TC. Yang et al. [
25] showed that ≥ 3 cups of tea consumption per day could be associated with a lower T2DM risk. Another meta-analysis evaluating the efficacy of green tea supplementation on nonalcoholic fatty liver disease indicated that green tea administration had a significantly decreasing effect on BMI, triglycerides, TC, and LDL-cholesterol levels [
26]. Results of a cross-sectional study supported that hot tea consumption might inversely be related to FBG in women, but not in men [
27]. Abasian et al. [
28] reviewed the role of medicinal plants in PCOS and suggested that consumption of herbal extracts containing phytoestrogens could result in decreases in blood testosterone, LH levels, and IR, but the blood progesterone and FSH levels were increased. The current meta-analysis has also documented the favorable effects of herbal tea drinking on weight, BMI, FBG, and FSH of PCOS women. Taking these findings together, individuals with low insulin sensitivity could benefit from frequent herbal tea consumption.
Green tea has catechins, which inhibit the degradation of norepinephrine; so, this neurotransmitter can prolong thermogenesis and fat metabolism [
29] and result in body weight reduction [
30]. Drinking of green tea could also decrease the appetite [
31,
32]. Furthermore, green tea can reduce the absorption of nutrients such as glucose by inhibiting of α-amylase, α-glucosidase, and glucose uptake in the intestine cells [
33,
34]. Marjoram herb could activate peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ; these PPARs play roles in improvement of the insulin sensitivity [
35]. Anti-androgenic properties of spearmint tea might reduce free testosterone levels in the blood and increase several female hormones including FSH. Its ability to increase the metabolism rate of androgens by inducing cytochrome P450 3A4.5. or the direct effect of spearmint on the synthesis of androgen hormones may cause this effect [
36]. Also, green tea may have an important anti-androgen effect due to its epigallocatechin content, which prevents the conversion of testosterone to dihydrotestosterone [
13], and marjoram is suggested to have mechanisms (yet to be investigated) that affect adrenal androgen production, other than the established activation of PPARs that improve hyperandrogenemia [
37].
As the strength of this study, all of the trials included in the meta-analysis were RCTs; so, the results had a high validity. Also, subgroup analyses based on potential covariates were carried out and the effect of herbal teas was investigated exclusively on PCOS women. The main limitation of the study was the small numbers of included studies and participants because the clinical trials that investigated the effects of herbal tea on PCOS women were rare. Another limitation was considering hirsutism, instead of PCOS, as an inclusion criterion for participants in a related trial [
36]. Another trial has also applied wood betony (Stachys lavandulifolia) as an herbal tea [
38]. So, these investigations could not be included in the present analysis. The type of placebo in control groups and tea in intervention groups were not the same in all eligible studies, also the length of intervention varied in the included studies. Although different herbs could have different effects, it was not possible for us to separately assess these differences, due to the small number of trials. These differences could be the source of heterogeneity. Moreover, we could not perform subgroup analysis, meta-regression, and dose-response analysis for most outcomes of interest due to few numbers of effect sizes. Inflammatory factors including interleukin 6, tumor necrosis factor α, and high-sensitivity c-reactive protein were reported as the PCOS outcomes only in one included trial [
15]; so, we could not evaluate the impact of tea supplementation on these biomarkers.
CONCLUSION
In conclusion, the current systematic review and meta-analysis revealed that herbal tea supplementation among a total of 235 women with PCOS (119 in the intervention and 116 in the control group) significantly decreased body weight, BMI, and serum FBG concentrations and increased FSH level in intervention group compared to control group. Whereas, herbal tea supplementation did not have significant effect on WHR, HC, WC, body fat, fasting insulin, glucose/insulin ratio, LH, total testosterone and DHEAS. Further large RCTs with herbal tea are recommended to affirm these findings.
Isfahan University of Medical Scienceshttps://doi.org/10.13039/501100003970
199253
NOTES
-
Funding: This study was supported by Isfahan University of Medical Sciences, Isfahan, Iran (Grant number: 199253).
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Saneei P, Abbasi E.
Investigation: Abbasi E, Hajhashemy Z, Askari G, Saneei P.
Methodology: Abbasi E, Hajhashemy Z, Askari G, Saneei P.
Writing - original draft: Abbasi E, Hajhashemy Z, Askari G, Saneei P.
Writing - review & editing: Abbasi E, Hajhashemy Z, Askari G, Saneei P.
SUPPLEMENTARY MATERIALS
Supplementary Figure 1
Quality assessment of included studies assessed the effects of herbal tea supplementation on polycystic ovarian syndrome in women.
cnr-13-201-s001.ppt
REFERENCES
- 1. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG practice bulletin No. 194: polycystic ovary syndrome. Obstet Gynecol 2018;131:e157-e171.
- 2. Balen AH, Conway GS, Kaltsas G, Techatrasak K, Manning PJ, et al. Polycystic ovary syndrome: the spectrum of the disorder in 1741 patients. Hum Reprod 1995;10:2107-2111.
- 3. Mulders AGMGJ, Laven JSE, Eijkemans MJC, de Jong FH, Themmen APN, et al. Changes in anti-Müllerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Hum Reprod 2004;19:2036-2042.
- 4. Navaratnarajah R, Pillay OC, Hardiman P. Polycystic ovary syndrome and endometrial cancer. Semin Reprod Med 2008;26:62-71.
- 5. Du D, Li X. The relationship between thyroiditis and polycystic ovary syndrome: a meta-analysis. Int J Clin Exp Med 2013;6:880-889.
- 6. Qin JZ, Pang LH, Li MJ, Fan XJ, Huang RD, et al. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol 2013;11:56.
- 7. Hoeger K. Obesity and weight loss in polycystic ovary syndrome. Obstet Gynecol Clin North Am 2001;28:85-97.
- 8. Liou TH, Yang JH, Hsieh CH, Lee CY, Hsu CS, et al. Clinical and biochemical presentations of polycystic ovary syndrome among obese and nonobese women. Fertil Steril 2009;92:1960-1965.
- 9. Harrison CL, Lombard CB, Moran LJ, Teede HJ. Exercise therapy in polycystic ovary syndrome: a systematic review. Hum Reprod Update 2011;17:171-183.
- 10. Raja-Khan N, Stener-Victorin E, Wu X, Legro RS. The physiological basis of complementary and alternative medicines for polycystic ovary syndrome. Am J Physiol Endocrinol Metab 2011;301:E1-E10.
- 11. Douglas CC, Gower BA, Darnell BE, Ovalle F, Oster RA, et al. Role of diet in the treatment of polycystic ovary syndrome. Fertil Steril 2006;85:679-688.
- 12. Hernandez-Rodas MC, Valenzuela R, Videla LA. Relevant aspects of nutritional and dietary interventions in non-alcoholic fatty liver disease. Int J Mol Sci 2015;16:25168-25198.
- 13. Grant P, Ramasamy S. An update on plant derived anti-androgens. Int J Endocrinol Metab 2012;10:497-502.
- 14. Chan CC, Koo MW, Ng EH, Tang OS, Yeung WS, et al. Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome--a randomized placebo-controlled trial. J Soc Gynecol Investig 2006;13:63-68.
- 15. Mombaini E, Jafarirad S, Husain D, Haghighizadeh MH, Padfar P. The impact of green tea supplementation on anthropometric indices and inflammatory cytokines in women with polycystic ovary syndrome. Phytother Res 2017;31:747-754.
- 16. Haj-Husein I, Tukan S, Alkazaleh F. The effect of marjoram (Origanum majorana) tea on the hormonal profile of women with polycystic ovary syndrome: a randomised controlled pilot study. J Hum Nutr Diet 2016;29:105-111.
- 17. Farhadian M, Barati S, Mahmoodi M, Barati Mosleh A, Yavangui M. Comparison of green tea and metformin effects on anthropometric indicators in women with polycystic ovarian syndrome: a clinical trial study. Journal of Reports in Pharmaceutical Sciences 2020;9:97-103.
- 18. Tehrani HG, Allahdadian M, Zarre F, Ranjbar H, Allahdadian F. Effect of green tea on metabolic and hormonal aspect of polycystic ovarian syndrome in overweight and obese women suffering from polycystic ovarian syndrome: a clinical trial. J Educ Health Promot 2017;6:36.
- 19. Grant P. Spearmint herbal tea has significant anti-androgen effects in polycystic ovarian syndrome. A randomized controlled trial. Phytother Res 2010;24:186-188.
- 20. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.4. 2023. cited 2023 Aug 22. Available from www.training.cochrane.org/handbook
- 21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-634.
- 22. Shen W, Pan Y, Jin B, Zhang Z, You T, et al. Effects of tea consumption on anthropometric parameters, metabolic indexes and hormone levels of women with polycystic ovarian syndrome: a systematic review and meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne) 2021;12:736867.
- 23. Chien YJ, Chang CY, Wu MY, Chen CH, Horng YS, et al. Effects of curcumin on glycemic control and lipid profile in polycystic ovary syndrome: systematic review with meta-analysis and trial sequential analysis. Nutrients 2021;13:684.
- 24. Payab M, Hasani-Ranjbar S, Shahbal N, Qorbani M, Aletaha A, et al. Effect of the herbal medicines in obesity and metabolic syndrome: a systematic review and meta-analysis of clinical trials. Phytother Res 2020;34:526-545.
- 25. Yang J, Mao QX, Xu HX, Ma X, Zeng CY. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open 2014;4:e005632.
- 26. Mansour-Ghanaei F, Hadi A, Pourmasoumi M, Joukar F, Golpour S, et al. Green tea as a safe alternative approach for nonalcoholic fatty liver treatment: a systematic review and meta-analysis of clinical trials. Phytother Res 2018;32:1876-1884.
- 27. Vernarelli JA, Lambert JD. Tea consumption is inversely associated with weight status and other markers for metabolic syndrome in US adults. Eur J Nutr 2013;52:1039-1048.
- 28. Abasian Z, Rostamzadeh A, Mohammadi M, Hosseini M, Rafieian-Kopaei M. A review on role of medicinal plants in polycystic ovarian syndrome: pathophysiology, neuroendocrine signaling, therapeutic status and future prospects. Middle East Fertil Soc J 2018;23:255-262.
- 29. Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am J Clin Nutr 1999;70:1040-1045.
- 30. Chantre P, Lairon D. Recent findings of green tea extract AR25 (Exolise) and its activity for the treatment of obesity. Phytomedicine 2002;9:3-8.
- 31. Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 2000;141:980-987.
- 32. Wellman PJ. Norepinephrine and the control of food intake. Nutrition 2000;16:837-842.
- 33. Johnston K, Sharp P, Clifford M, Morgan L. Dietary polyphenols decrease glucose uptake by human intestinal Caco-2 cells. FEBS Lett 2005;579:1653-1657.
- 34. Matsumoto N, Ishigaki F, Ishigaki A, Iwashina H, Hara Y. Reduction of blood glucose levels by tea catechin. Biosci Biotechnol Biochem 1993;57:525-527.
- 35. Bhatia V, Viswanathan P. Insulin resistance and PPAR insulin sensitizers. Curr Opin Investig Drugs 2006;7:891-897.
- 36. Akdoğan M, Tamer MN, Cüre E, Cüre MC, Köroğlu BK, et al. Effect of spearmint (Mentha spicata Labiatae) teas on androgen levels in women with hirsutism. Phytother Res 2007;21:444-447.
- 37. Haj-Husein I, Tukan S, Alkazaleh F. The effect of marjoram (Origanum majorana) tea on the hormonal profile of women with polycystic ovary syndrome: a randomised controlled pilot study. J Hum Nutr Diet 2016;29:105-111.
- 38. Jalilian N, Modarresi M, Rezaie M, Ghaderi L, Bozorgmanesh M. Phytotherapeutic management of polycystic ovary syndrome: role of aerial parts of wood betony (Stachys lavandulifolia). Phytother Res 2013;27:1708-1713.