ABSTRACT
Nutrition support is an essential aspect of treatment after bariatric surgery (BS). A high-protein diet with an intake of up to 1.5 g/kg of ideal body weight (IBW) per day is recommended to minimize loss of lean body mass after BS. However, protein intake recommendations may need to be adjusted for patients with compromised renal function, necessitating an individualized approach tailored to each patient’s clinical status. This case report aimed to demonstrate nutritional evaluation, education, and counseling for a male patient with chronic kidney disease (CKD) who underwent BS one year after surgery. Following BS, the patient adhered to the standard Seoul National University Hospital BS diet protocol. Considering his postoperative renal function, protein requirement was set at 1.0 g/kg of IBW. A total of 10 individualized nutritional counseling sessions were conducted according to renal function and complications. One year after BS, he successfully lost weight with % excess weight loss of 93%, maintained CKD stage 3, reduced prescription of oral hypoglycemic agent, improved glycated hemoglobin levels, and improved eating habits significantly. Thus, individualized nutrition intervention is important for supporting patients with CKD to reach their goal weight after BS, improve nutritional status, and prevent post-operative complications.
-
Keywords: Bariatric surgery; Kidney disease; Obesity management; Diet therapy
INTRODUCTION
Obesity is associated with both risk and progression of chronic kidney disease (CKD). Bariatric surgery (BS) is now recognized as a safe and effective treatment option for obese CKD patients. Weight loss, improved glycemic control, and antihypertensive effects following BS have been shown to confer renal protective benefits [
1]. Consequently, CKD is no longer considered a contraindication for BS. Postoperative nutritional management is essential for patient undergoing BS. Goals of nutrition support after BS are to minimize loss of lean body mass, alleviate gastrointestinal symptoms, and prevent nutritional deficiencies including micronutrient deficits [
2]. According to the 2018 guidelines for bariatric metabolic surgery, regular pre- and post-surgery nutritional counseling by experts is recommended. Target energy after surgery along with micronutrient supplementation is 1,000 to 1,400 kcal per day with 60 to 80 g of protein per day or up to 1.5 g/kg of ideal body weight (IBW) [
3]. However, for patients with reduced renal function, nutritional requirements and interventions may vary. The recommended protein intake after BS for CKD stages 3–5 without dialysis is 0.8 to 1 g/kg of IBW [
4]. It is 0.6 to 0.8 g/kg of IBW for long term care transitioning to solid foods for patients with CKD stages 4–5 after sleeve gastrectomy [
5,
6]. This case report provides information on nutrition support and intervention for a CKD patient undergoing BS over the course of one year.
CASE
A 64-year-old male patient with underlying diseases including diabetes mellitus, CKD, hypertension, and colostomy (due to rectal cancer, currently no evidence of the disease) was admitted to Seoul National University Hospital (SNUH) for sleeve gastrectomy. The patient reported weight gain after his previous surgery for rectal cancer and multiple unsuccessful attempts at weight loss. He also reported that he consumed 2–3 irregular meals a day, had inconsistent meal time, and preferred sugary foods such as fruit, bread, noodles, and drinks. The patient’s preoperative anthropometric measurements were as follows: height, 169 cm; weight, 102.2 kg; body mass index (BMI), 35.5 kg/m
2; and IBW 62.8 kg. The patient received nutritional counseling a total of 10 times over the course of one year, with details summarized in
Figure 1. This case report was approved by the Institutional Review Board (IRB) of SNUH (IRB No. 2409-079-1570). This study was conducted with the patient's written informed consent.
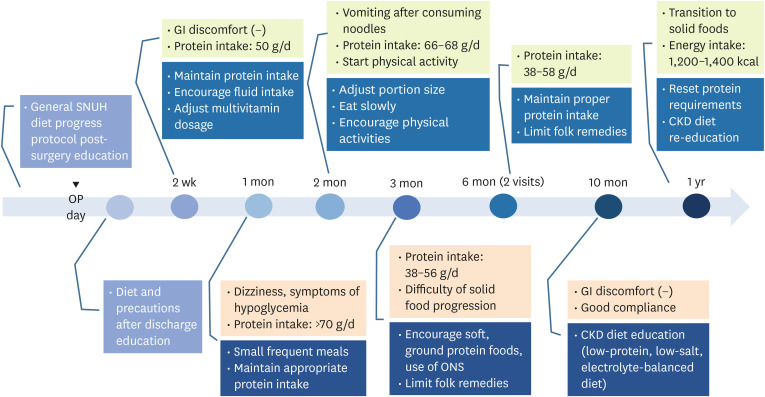
Figure 1
Summary of nutritional care and education outcomes during the intervention period.
OP, operation; SNUH, Seoul National University Hospital; GI, gastrointestinal; CKD, chronic kidney disease; ONS, oral nutritional supplement.
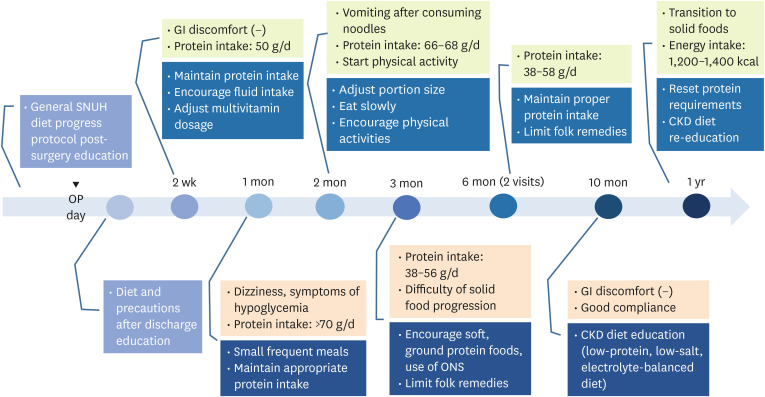
During hospitalization (POD #0–1 week)
Perioperative nutritional counseling was provided, focusing on dietary goals after surgery, meal progression stages, post-surgery diet guidelines and precautions. On the second day after surgery, he began consuming sips of water and clear liquids. He was instructed to incorporate protein powder into liquid meals and take a daily multivitamin. The general dietary progression protocol for post BS at SNUH is outlined in
Table 1.
Table 1General Seoul National University Hospital post-bariatric surgery dietary progression protocol
Table 1
|
Postoperative day |
Diet type |
Dietary composition |
Energy and protein |
|
#3–4 |
Clear-full liquid diet |
- Rice porridge 1/2 bowl + ONS 100 mL + protein powder 30 g/d |
Energy: 500 kcal/d |
|
Protein: 40 g/d |
|
#5–13 |
Full liquid diet |
- ONS 100 mL/portion, protein powder 30 g/d |
Energy: 600 kcal/d |
|
- Plain yogurt can be consumed in small amounts |
Protein: < 50 g/d |
|
#14–28 |
Pureed soft diet |
- Adjust intake amounts for soft food |
Energy: 600–700 kcal/d |
|
- Solid foods up to 1/3 cup, liquid foods up to 1/2 cup |
Protein: < 60 g/d |
|
- Prioritize protein intake (30 g/d) |
|
|
#28–42 |
Soft solid diet |
- Adjust intake amounts |
Energy: 800 kcal/d |
|
- Solid foods up to 1/2 cup, liquid foods up to 1 cup |
Protein: 60–70 g/d |
|
- Prioritize protein intake (30 g/d) |
|
|
#43– |
Regular, balanced diet |
- Limit 1 intake to 1 cup per meal |
Energy: 1,000 kcal/d |
|
- Prioritize protein intake (gradually reduce protein powder) |
Protein: < 80 g/d |
POD #2 weeks
Nutritional counseling was provided during the first outpatient visit after surgery. At this time, the patient's BMI was 34.1 kg/m
2 with a weight of 97.3 kg. His energy intake ranged from 700–800 kcal daily, with approximately 50 g of protein consumed daily. He reported no gastrointestinal discomfort such as reflux, vomiting, or early satiety. Considering early postoperative period and renal function, protein requirement was set at 1.0 g/kg of IBW. The patient’s serum uric acid levels had increased. According to a study assessing uric acid levels after BS, there was a statistically significant increase at one-week post-surgery, followed by a substantial decrease at one and 3 months. This can be attributed to metabolic changes due to rapid weight loss, difficulties in initial fluid intake, and a protein-rich diet, all of which might have contributed to elevated serum uric acid levels [
7]. Upon evaluating the patient’s protein and fluid intake, we found that his protein consumption during the liquid diet phase was appropriate. Therefore, we imposed no restrictions. However, his fluid intake was inadequate compared to recommended levels, prompting us to encourage increased consumption. Regarding vitamin and mineral supplementation, he was advised to take half the usual dosage of multivitamins for post-BS patients, considering renal function. In addition, we assessed the patient’s psychological state post-surgery and encouraged him to set his own short- and long-term weight loss goals for motivation.
A nutritional reassessment was conducted, revealing a BMI of 33.4 kg/m2, a weight of 95.4 kg, an energy intake of 900 kcal, and a protein intake of over 70 g (> 1.1 g/kg of IBW). We re-educated the recommended protein intake. He reported experiencing dizziness and symptoms of hypoglycemia. To prevent hypoglycemia, he was advised to consume foods in small amount frequently and to avoid excessively restricting carbohydrates. Additionally, he was asked to talk about positive changes after the surgery. He was given appropriate praise and encouragement. He promised the registered dietitian that he would return for further nutritional counseling.
POD #2 months
At the 2-month follow-up, nutritional counseling was provided. The patient's BMI was 32.5 kg/m2 with a weight of 92.95 kg, an energy intake of 1,000 kcal, and a protein intake ranging from 66 to 68 g. He reported vomiting after consuming noodles likely due to a rapid eating speed. He increased meal size and the variety of foods. He was advised to control his portion size and eat slowly. In addition, he began physical activities. He was satisfied with positive changes in his life since surgery.
POD #3 months
Three months after surgery, the patient’s weight was 87 kg with a BMI of 30.5 kg/m2. A nutritional reassessment revealed that he was consuming less than the recommended protein intake, with a minimum of 0.6 g/kg of protein. He was having difficulty transitioning to solid foods. To ensure adequate protein intake, we recommended incorporating soft protein-rich foods. He also reported drinking celery juice and green onion juice instead of water. Folk remedies were limited considering his renal function.
POD #6 months
His nutritional status was reevaluated twice at 6 months. At the first visit, the patient’s weight was 81.3 kg with a BMI of 28.5 kg/m2. At the second visit, his weight was 80 kg, which showed a good reduction rate. However, there was a discrepancy in protein intake, which was corrected. He was encouraged to consume within the recommended intake.
POD #1 year
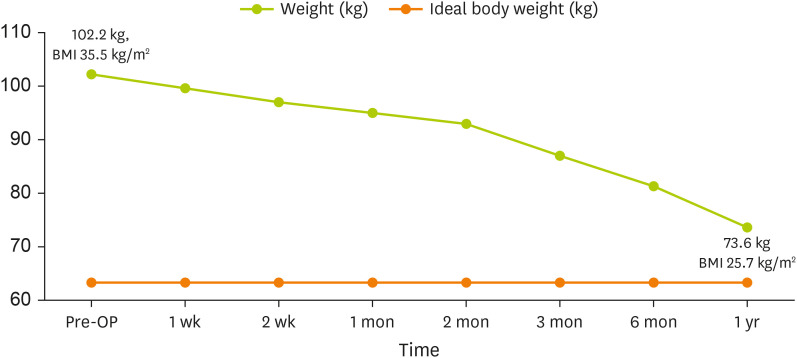
At approximately one year after surgery, the patient achieved a weight of 73.6 kg (BMI: 25.7 kg/m
2) (
Figure 2). He completely transitioned to a regular diet. We recommended a protein intake of 0.8 g/kg and a low salt diet, considering long-term care needs for a CKD patient. His energy requirement was set at 1,600 kcal (IBW × 25 kcal) for weight maintenance. Pre- and post-operative laboratory measurements are shown in
Table 2.
Figure 2
Body weight changes after bariatric surgery.
BMI, body mass index; OP, operation.
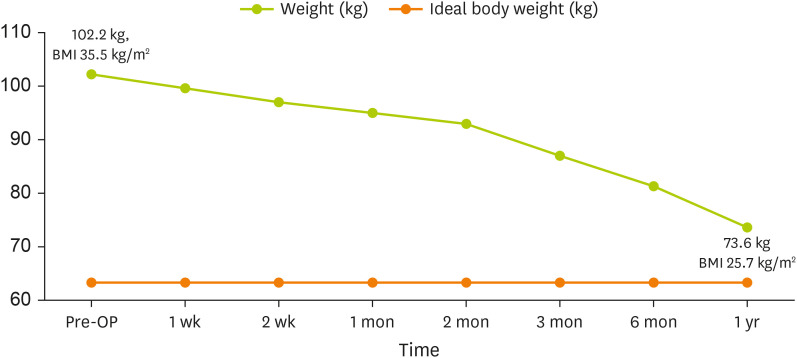
Table 2Pre- and post-operative laboratory measurements
Table 2
|
Variables |
Normal range |
Pre-OP |
OP day |
POD 2 wk |
POD 1 mon |
POD 2 mon |
POD 3 mon |
POD 6 mon |
POD 1 yr |
|
Albumin (g/dL) |
3.3–5.2 |
4.4 |
3.9 |
4.4 |
- |
4.5 |
4.5 |
4.5 |
4.9 |
|
Hemoglobin (g/dL) |
13–17 |
11.7 |
11.4 |
12.3 |
11.7 |
13 |
13.5 |
13 |
14.7 |
|
Hematocrit (%) |
39–52 |
36.6 |
34.9 |
37.8 |
34.5 |
38.7 |
40.2 |
38.8 |
42.7 |
|
BUN/Cr (mg/dL) |
10–26/0.70–1.40 |
32/1.87 |
23/1.64 |
43/1.89 |
- |
28/1.96 |
21/1.97 |
30/1.75 |
21/2.20 |
|
eGFR (CKD-EPI Cr) |
- |
37.2 |
43.5 |
36.7 |
- |
34.9 |
34.6 |
40 |
30.3 |
|
Uric acid (mg/dL) |
3.0–7.0 |
6.4 |
6.0 |
8.0 |
- |
6.7 |
8.0 |
7.7 |
8.4 |
|
HbA1c (%) |
4.0–6.4 |
6 |
- |
6.1 |
- |
5.5 |
- |
6.1 |
5.8 |
|
Fasting glucose (mg/dL) |
70–110 |
107 |
98 |
111 |
- |
109 |
99 |
116 |
94 |
|
PTH (pg/mL) |
8–76 |
28 |
- |
- |
17 |
23 |
22 |
14 |
15 |
|
Iron (mcg/dL) |
50–170 |
81 |
- |
- |
- |
120 |
- |
86 |
- |
|
K (mmol/L) |
3.5–5.5 |
5.7 |
5.1 |
4.6 |
4.0 |
4.4 |
4.7 |
4.7 |
5.2 |
|
Phosphorus (mg/dL) |
2.5–4.5 |
3.2 |
2.1 |
3.0 |
- |
3.5 |
2.7 |
2.8 |
3.9 |
|
25(OH) vitamin D (ng/mL) |
30–100 |
19.8 |
- |
- |
33.0 |
37.3 |
25.4 |
42 |
54.8 |
|
Vitamin B12 (pg/mL) |
197–771 |
99.36 |
- |
- |
- |
208.1 |
- |
149.8 |
249.8 |
|
Folate (ng/mL) |
3.89–26.8 |
3.81 |
- |
- |
- |
13.54 |
- |
21.64 |
37.88 |
DISCUSSION
The patient lost approximately 30 kg at one year after BS, achieving 93% excess weight loss and demonstrating improvement in complications. Among oral hypoglycemic agents prescribed prior to surgery, pioglitazone was discontinued. Only metformin and gemigliptin were taken at one year after surgery, with prescription doses reduced. Glycated hemoglobin improved by 0.2%. His prescription doses of antihypertensive drugs and antianginal drugs were also reduced.
The patient’s renal function remained CKD stage 3 at one year after surgery. Chang et al. [
8] have studied the association between BS and improvements in kidney outcomes. They reported that patients who underwent BS had a 58% lower risk for decreased estimated glomerular filtration rate of 30% or more compared to a matched cohort control group who did not undergo surgery [
8]. Additionally, a group of obese pre-dialysis patients with CKD stages 3–5 who underwent weight loss surgery was found to have a 79% lower 5-year mortality risk compared to a group of similar adults who did not [
9]. While our patient’s renal function did not significantly improve at one year after surgery, it is important to consider that obesity and metabolic syndrome are potential contributors to end-stage renal failure [
10]. Therefore, the potential for preventing renal function decline related to obesity in the future may be promising.
Several studies have reported preoperative micronutrient deficiencies in patients undergoing BS [
11,
12]. Preoperative vitamin D, iron, folic acid, and vitamin B
12 levels were also deficient in 75.2%, 42.6%, 28.8%, and 8.5% of patients undergoing BS, respectively [
12]. Therefore, expert groups emphasize that pre-nutritional evaluation, including micronutrients, should be performed before surgery. Our patient was also observed to be deficient in vitamin D, folic acid, and vitamin B
12 before surgery, which improved after taking multivitamin and receiving postoperative nutrition care.
His eating habits also improved significantly. He had an irregular eating pattern and preferred drinks and sugary snacks before. However, he learned to stop eating when he felt full, consume appropriate amounts, limit high-calorie foods, and consume balanced meals through several rounds of nutritional counseling.
However, this case report has limitations. Body composition was not assessed through dual energy X-ray absorptiometry or bioelectrical impedance analysis during the perioperative period. A more accurate nutritional evaluation could have been made before and after surgery.
The average excess weight loss rate after sleeve gastrectomy has been reported to be 59.3% at one year, 59% at 2 years, 54.7% at 3 years, 52.3% at 4 years, and 52.4% after 5 years [
13]. In the present study, it was 93% after 1 year. At 2 years and 4 months after the surgery, the patient had an excess weight loss rate of 98% without weight gain based on medical records. Endevelt et al. [
14] found that patients who experienced consultation with a dietician after BS had a 5% higher rate of BMI reduction. Additionally, postoperative monitoring and dietary assessment are important for weight loss and maintenance. Structured nutritional counseling can contribute to significant weight reduction [
14]. This case report showed similar results. Over the course of one year, 10 nutritional counseling sessions were conducted, resulting in substantial weight loss and positive clinical results.
Therefore, continuous nutritional re-evaluation and counseling are critical factors for achieving clinical outcomes such as reaching target weight, improving nutritional status, and preventing complications after BS. In addition, nutritional requirements of patients with CKD who have undergone BS should be individualized based on the patient’s clinical condition. Evidence-based, flexible nutritional interventions and education tailored to compliance and clinical progress are needed.
NOTES
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Choi Y, Ju DL.
Data curation: Choi Y.
Investigation: Choi Y.
Writing - original draft: Choi Y.
Writing - review & editing: Song J, Lim JH, Ju DL.
REFERENCES
- 1. Docherty NG, le Roux CW. Bariatric surgery for the treatment of chronic kidney disease in obesity and type 2 diabetes mellitus. Nat Rev Nephrol 2020;16:709-720.
- 2. Sherf Dagan S, Goldenshluger A, Globus I, Schweiger C, Kessler Y, et al. Nutritional recommendations for adult bariatric surgery patients: clinical practice. Adv Nutr 2017;8:382-394.
- 3. Guideline Committee for Metabolic and Bariatric Surgery, Korean Society for Metabolic and Bariatric Surgery. Korean Society of Obesity and Metabolic Surgery guidelines for bariatric surgery. J Metab Bariatr Surg 2018;7:1-21.
- 4. Ben-Porat T, Weiss-Sadan A, Rottenstreich A, Sherf-Dagan S, Schweiger C, et al. Nutritional management for chronic kidney disease patients who undergo bariatric surgery: a narrative review. Adv Nutr 2019;10:122-132.
- 5. Kukla A, Kudva YC, Navratil P, Sahi SS, Benzo RP, et al. Management of patients with kidney disease undergoing bariatric surgery: a multidisciplinary approach. Mayo Clin Proc 2024;99:445-458.
- 6. Majorowicz RR, Attia A, Bamlet HM, Clegg DJ, Diwan T, et al. Nutritional considerations for patients with renal failure undergoing sleeve gastrectomy. J Ren Nutr 2024;34:76-86.
- 7. Li M, Liu Y, Zeng N, Liu J, Bian S, et al. Alterations in the serum urate concentrations after bariatric surgery: a short-term prospective observational study. Obes Surg 2021;31:1688-1695.
- 8. Chang AR, Chen Y, Still C, Wood GC, Kirchner HL, et al. Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int 2016;90:164-171.
- 9. Coleman KJ, Shu YH, Fischer H, Johnson E, Yoon TK, et al. Bariatric surgery and risk of death in persons with chronic kidney disease. Ann Surg 2022;276:e784-e791.
- 10. Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures – 2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg Obes Relat Dis 2020;16:175-247.
- 11. Peterson LA, Cheskin LJ, Furtado M, Papas K, Schweitzer MA, et al. Malnutrition in bariatric surgery candidates: multiple micronutrient deficiencies prior to surgery. Obes Surg 2016;26:833-838.
- 12. Ben-Porat T, Weiss R, Sherf-Dagan S, Nabulsi N, Maayani A, et al. Nutritional deficiencies in patients with severe obesity before bariatric surgery: what should be the focus during the preoperative assessment? J Acad Nutr Diet 2020;120:874-884.
- 13. Gagner M, Deitel M, Erickson AL, Crosby RD. Survey on laparoscopic sleeve gastrectomy (LSG) at the fourth international consensus summit on sleeve gastrectomy. Obes Surg 2013;23:2013-2017.
- 14. Endevelt R, Ben-Assuli O, Klain E, Zelber-Sagi S. The role of dietician follow-up in the success of bariatric surgery. Surg Obes Relat Dis 2013;9:963-968.