ABSTRACT
Critically ill trauma patients generally show good nutritional status upon initial hospitalization. However, they have a high risk of malnutrition due to hyper-metabolism during the acute phase. Hence, suitable nutritional support is essential for the optimal recovery of these patients; therefore, outcomes such as preservation of fat-free mass, maintenance of immune functions, reduction in infectious complications, and prevention of malnutrition can be expected. In this report, we present the experience of a patient subjected to 40 days of nutritional interventions during postoperative intensive care unit (ICU) care. Although the patient was no malnutrition at ICU admission, enteral nutrition (EN) was delayed for > 2 weeks because of several postoperative complications. Subsequently, while receiving parenteral nutrition (PN), the patient displayed persistent hypertriglyceridemia. As a result, his prescription of PN were converted to lipid-free PN. On postoperative day (POD) #19, the patient underwent jejunostomy and started standard EN. A week later, the patient was switched to a high-protein, immune-modulating formula for postoperative wound recovery. Thereafter, PN was stopped, while EN was increased. In addition, because of defecation issues, a fiber-containing formula was administered with previous formula alternately. Despite continuous nutritional intervention, the patient experienced a significant weight loss and muscle mass depletion and was diagnosed with severe malnutrition upon discharge from the ICU. To conclude, this case report highlights the importance of nutrition interventions in critically ill trauma patients with an increased risk of malnutrition, indicating the need to promptly secure an appropriate route of feeding access for active nutritional support of patients in the ICU.
-
Keywords: Critical illness; Wounds and injuries; Enteral nutrition; Malnutrition
INTRODUCTION
Trauma occurs at a relatively younger age than other diseases, and inadequate initial management can lead to a high mortality. Moreover, unpredicted physical damage causes hemodynamic, inflammatory, and metabolic changes. As a result of hemodynamic changes, while blood flow to the skeletal muscle increases due to tissue damage and hemorrhage, blood flow to the mucosa decreases as a compensatory mechanism, inducing bacterial translocation. As a result of the inflammatory response, cytokine release causes multi-organ failure. Then, because of metabolic changes, blood sugar levels rise due to the actions of catabolic hormones, increasing the risk of infection. Furthermore, proteolysis occurs simultaneously, causing the body to use amino acids as an energy source, creating a vicious cycle. Moreover, insufficient nutritional supply accelerates proteolysis, decreases immune functions, and delays wound healing [
1].
As a result of the abovementioned, appropriate nutritional support during treatment is critical to minimizing catabolic stress and loss of fat-free mass associated with negative nitrogen balance. It can also decrease the mortality and hospitalization duration. The American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines [
2] also strongly recommend enteral nutrition (EN) over parenteral nutrition (PN) as a feeding access route. Because by using EN can utilize the nutrients more efficiently to preserve immune functions and prevent mucosal atrophy. In hemodynamically unstable patients, EN is recommended after shock resuscitation. Depending on the severity of the trauma, a study recommended the supply of 20–35 kcal/kg/d of energy and 1.2–2.0 g/kg/d of protein. Meanwhile, in severe trauma patients, the use of an immune-modulating formula containing arginine and fish oil should be considered. According to the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines [
3], enteral glutamine supplementation is recommended for as long as ≥ 15 days when complicated wound healing is necessary. Such immunonutrition positively affects infection reduction and wound recovery in critically ill trauma patients.
Therefore, this case report provides information about the nutritional interventions for a critically ill trauma patient admitted to the intensive care unit (ICU) of our hospital. We also discuss the effective methods of nutrition support implemented for the patient’s quick recovery. Institutional Review Board approval was obtained from Gangnam Severance Hospital (approval number 3-2022-0044).
CASE
A 28-year-old male patient with no distinctive medical history was hospitalized at the emergency room of the Gangnam Severance Hospital after falling from the 4th floor of a building. Preliminary examinations revealed liver and spleen lacerations as well as multiple bone fractures (femur, pelvic bone, etc.) after which a chest tube was inserted for the treatment of pneumothorax and hemothorax. After surgery, the patient was admitted to the ICU for mechanical ventilation. At admission, the patient’s height was 175 cm, weight was 70 kg, and body mass index (BMI) was 22.9 kg/m
2. However, his initial Acute Physiology and Chronic Health Evaluation (APACHE) II score was 31. The investigation also revealed that although the patient showed no signs of malnutrition, he was at a high risk of malnutrition because of the severity of disease, a Nutritional Risk Screening (NRS) 2002 score of 3 points, and a Nutrition Risk in Critically Ill (NUTRIC) score of 5 points. Therefore, during 40 days of ICU stay, the patient was referred to the nutrition support team (NST) at postoperative days (PODs) #0, #6, #19, #25, and #39 to assist with diet interventions.
Table 1 summarizes the nutritional interventions administered to the patient.
Table 1 Summary of nutrition management during ICU stay
Table 1
|
LOS |
POD |
Weight |
Nutrition management |
|
#2 |
#0 |
70 kg |
ICU admission, no nutrition supply, start PN on POD#3 |
|
#8 |
#6 |
70 kg |
Change to lipid free PN, add intravenous amino-acids |
|
#17 |
#15 |
73.7 kg |
Change to lipid free PN, add intravenous vitamins/trace elements |
|
#21 |
#19 |
73 kg |
Strat EN, stop PN |
|
#27 |
#25 |
67.8 kg |
Switch to the high protein, immune-modulating EN formula |
|
#41 |
#39 |
55.8 kg |
Administer the fiber-containing formula and the previous formula alternately |
POD #0
Following surgery for multiple bone fractures, the patient was admitted to the ICU and referred to the NST for optimal nutritional interventions. However, because of several complications, such as restricted postural change, unstable vital signs, and large gastric residual volume (GRV) after an EN attempt by nasogastric (NG) tube, the patient was kept mostly on PN for nutritional supply.
POD #6, POD #15
Subsequently, the patient was referred to the NST for PN adjustment. The nutrition requirements was calculated using the Penn State equation, with 2,200 kcal (considering fever) of energy and 105 g of protein. However, because the patient was receiving a high dose of propofol at 18 mL/h and had a triglyceride level of 699, a lipid-free PN formula was recommended. Additional interventions of intravenous (IV) amino acids infusion, IV multivitamins infusion, and IV trace elements infusion were provided simultaneously.
POD #19
Following jejunostomy, the patient was referred to the NST again to begin EN. The initial plan was to maintain PN and start continuous feeding of a 400 kcal fiber-free formula. However, the patient began vomiting after EN. As a result, antiemetics were administered, and an increase in EN to 20 mL/h was suggested.
POD #25
After the mandibular fracture surgery, the patient was referred to the NST for follow-up (F/U). First, nutritional requirements were modified to 2,000 kcal of energy and 95 g of protein (1.2–1.5 g/kg). Then, EN was increased to 800 kcal (40 mL/h) providing 40% of the nutrition requirements and PN with IV amino acids providing 20%. However, to supply immunonutrition for postoperative wound recovery and increase in the protein supply was recommended by switching to a high-protein, immune-modulating formula with 1,200 kcal (60 mL/h) of energy. The next day, PN was discontinued, and the prescription for EN was changed to provide 60% of the nutrition requirements. Although the modifications resulted in loose feces being defecated approximately twice daily, digestion was satisfactory.
POD #31
After undergoing reconstructive surgery for the mandibular fracture, the patient slowly recovered as he became mentally alert with no more sedatives and was weaned off the mechanical ventilator. However, with a gradual increase in EN up to 1,800 kcal (90% of the requirements), the frequency of loose stools increased to 6 times daily. Subsequent test results for Clostridium difficile were negative, but loose stools persisted. Therefore, the patient was administered antidiarrheal agents.
POD #39
Again, the patient was referred to the NST for F/U. He lost weight significantly, from 70 kg at POD #0 to 55.8 kg at POD #39, indicating severe malnutrition as evidenced by lean muscle mass and body fat depletion. Therefore, nutrition requirements were modified to 1,900 kcal (30–35 kcal/kg/d) and 90 g protein (1.3–1.6 g/kg/d). However, the frequency of loose stools increased, prompting the administration of the fiber-containing formula and the high-protein, immune-modulating formula alternately. The patient was started on oral sips of liquid diet the next day, and nutritional interventions were finished as the patient was transferred to the general ward.
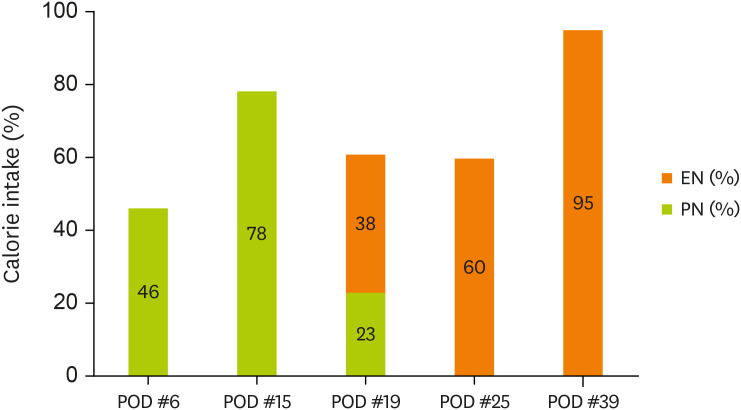
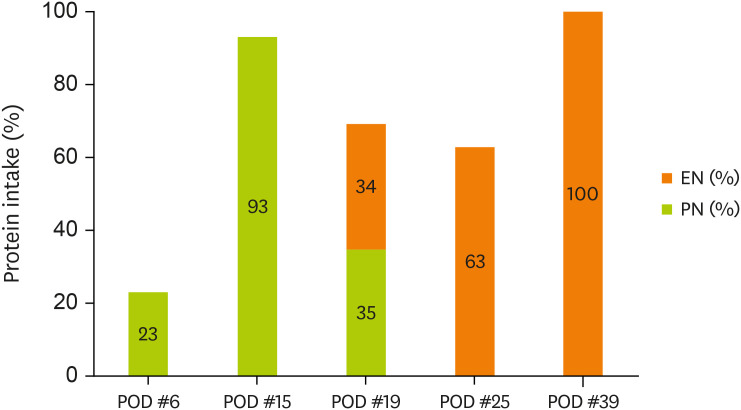
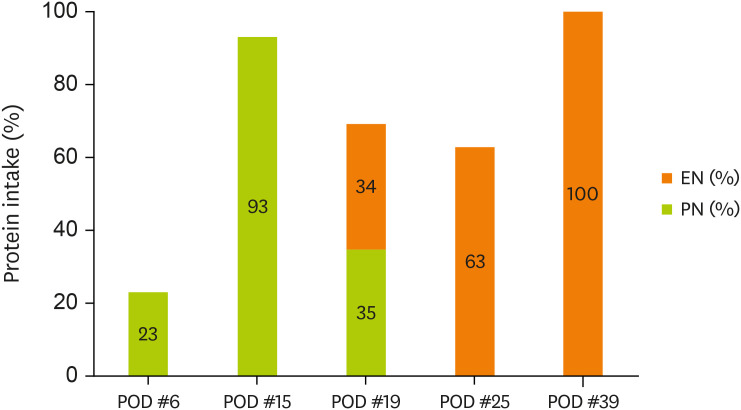
Figures 1 and
2 display the changes in the supplied nutrient amount (%) versus the required amount of nutrition during ICU admission.
Figure 1
Change in energy intake through EN and PN.
EN, enteral nutrition; PN, parenteral nutrition; POD, postoperative day.
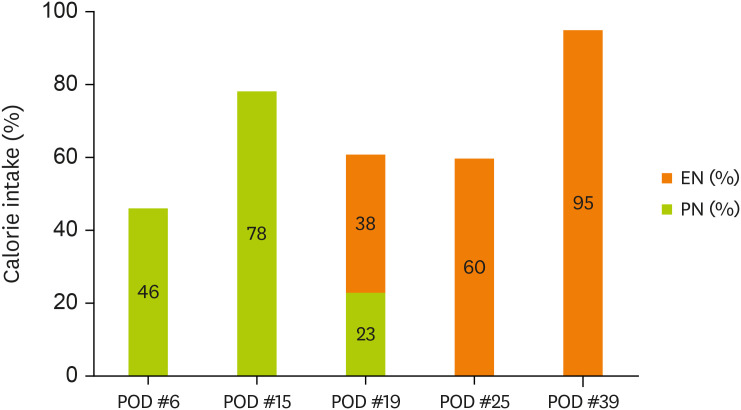
Figure 2
Change in protein intake through EN and PN.
EN, enteral nutrition; PN, parenteral nutrition; POD, postoperative day.
DISCUSSION
Following significant physical damage, the body of trauma patients sustains reduced oxygen supply to body tissue in order to maintain homeostasis. This shortage increases heart rate and ventilation volume per minute, causing the body to quickly enter into a catabolic state. Insufficient nutrition support during this period can result in significant weight loss [
1]. Despite the nutritional interventions, this patient lost 14 kg (20%) over 40 days. Therefore, we discuss the cause of this weight loss and consider potential methods for improvement.
The first potential reason is the delay in starting EN after ICU admission. Although enteral access was established early with an NG tube, immediately starting EN was difficult because of his unstable vital status and GRV. Therefore, EN was attempted on POD #6, but the GRV continued to exceed the supply volume. As a result, PN continued to be the main source of nutrition supply. Subsequently, EN was only made possible after a jejunostomy was performed on POD #19. McCartt et al. [
4] developed a protocol to enhance EN supply in critically ill trauma or surgical patients and proposed three main elements. First, until surgery, EN is recommended for patients with secure airways. This step excludes patients undergoing airway manipulation or patients in a prone position. Second, for patients with a high risk of malnutrition, a post pyloric tube is recommended to secure enteral access within 48 hours. The third is a volume-based feeding protocol. The author also revealed that in the protocol implementation group, the rate of reaching > 80% of the energy requirements through EN should result in a significant increase of 10.2%. In contrast to the above protocol, the patient in our study was only subjected to volume-based feeding. Hence, adopting a post pyloric tube was emphasized over gastric access to avoid discontinuation of EN-dependent gastrointestinal side effects. Thus, given that active feeding access was possible for the patient after jejunostomy, we believe that the fasting period could have been reduced if a post pyloric tube had been secured sooner.
The second point is that PN supply was stopped prematurely. The ASPEN guidelines [
2] recommend the PN should be reduced and finally discontinued when the patient is receiving >60% of target energy requirements from EN. In this case, PN was halted immediately after 40% of the nutrition requirements from EN was met following jejunostomy. However, we believe that severe malnutrition could have been prevented if the PN access had been maintained until the nutrition requirements reached > 60% and if the time taken to reach the nutrition requirement had been reduced.
Third, the appropriate amount of protein to be supplied was not adequately evaluated. Although the patient’s kidney function was normal for most of his ICU stay, we could not evaluate the adequacy of the protein quantity supplied based on nitrogen balance after hemodynamic stabilization. Accurate calculation of nutrition requirements in critically ill patients is extremely challenging. Nevertheless, indirect calorimetry is the gold standard, despite numerous obstacles to its use in actual clinical practice. As recommended by several guidelines, the weight-based equation is the most commonly used, but it is only an estimate, and there may be a gap between the actual amount of nutrition required by the patient and the estimated requirements. For example, according to the trauma nutrition guidelines [
5], while the recommended energy requirements for a critically ill trauma patient is 25–30 kcal/kg/d or Harris-Benedict equation*1.3–1.5, the recommended protein requirements is 1.5–2.0 g/kg/d. However, depending on the severity of the trauma, the ASPEN guidelines [
2] recommend an energy supply of 20–35 kcal/kg/d and a protein supply of 1.2–2.0 g/kg/d. Notably, trauma and surgical patients generally lose 110 g of protein/d and 150 g of fat-free mass/d owing to their metabolic state and wounds [
1]. Hence, continuous monitoring to assess the appropriate protein requirements for the patients is essential.
A final point to consider is the inability to prevent deterioration of nutritional status, including weight loss, despite using a high-protein, immune-modulating formula. Thus, the ASPEN guidelines [
2] recommend early enteral feeding with a high-protein polymeric diet for critically ill trauma patients. An immune-modulating formula containing arginine and fish oil can also be considered for severe trauma patients. Notably, arginine increases nitric oxide production and positively affects immune functions and infection reductions. Furthermore, the omega-3 fatty acids, eicosapentaenoic acid and docosahexaenoic acid, replace omega-6 fatty acids in the membrane of immune cells, reducing their overall inflammation [
5]. However, the synergistic effect can only be observed with an enteral-modulating formula combining arginine and fish oil to overcome the modulation of myelosuppression cells. A study reported that 5 days after starting EN, 0.2–0.3 g/kg/d of enteral glutamine supplementation is recommended for critically ill trauma patients [
3]. Alternatively, glutamine maintains the intestinal epithelial barrier and positively affects the immune function of critically ill patients. Additionally, another study reported that simultaneously administering antioxidants and glutamine can shorten the time required for wound closure [
6]. Therefore, based on the several guidelines’ recommendation, the standard formula was replaced with a high antioxidant and omega-3 fatty acid formula. Nonetheless, the product used in our study did not contain glutamine and arginine. Hence, expecting a synergistic effect is difficult. However, for a quick post-op recovery, the supply of an adequate combination of immunonutrients such as omega-3 fatty acid, arginine and glutamine is essential. Therefore, a clinical dietitian’s role is to be interested in such products and provide accurate information to multidisciplinary members.
In conclusion, because critically ill trauma patients are at a high risk of malnutrition and can also experience a depletion of fat-free mass in a short period of time owing to metabolic changes, active nutritional interventions are essential. In accordance with evidence-based guidelines, an adequate nutrition supply can help to maintain immune function and wound healing in critically ill trauma patients. Furthermore, continued nutrition support after ICU discharge can reduce the length of hospital stay and improve patient quality of life.
NOTES
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Kim SH, Kim SJ, Kim W.
Writing - original draft: Kim SH.
Writing - review & editing: Kim SH.
REFERENCES
- 1. Mueller CM, Lord LM, Marian M, McClave S, Miller SJ. Trauma, surgery, and burns. In Evans DC, Collier BR, eds, ddThe ASPEN adult nutrition support core curriculum. 3rd ed. Silver Spring (MD): American Society for Parenteral and Enteral Nutrition; 2017, pp 473-484.
- 2. McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice TW, Cresci GA, Gervasio JM, Sacks GS, Roberts PR, Compher C. Society of Critical Care Medicine. American Society for Parenteral and Enteral Nutrition. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JPEN J Parenter Enteral Nutr 2016;40:159-211.
- 3. Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C, Preiser JC, van Zanten AR, Oczkowski S, Szczeklik W, Bischoff SC. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 2019;38:48-79.
- 4. McCartt J, Loszko A, Backes K, Cunningham K, Evans S, Draughon M, Sachdev G. Improving enteral nutrition delivery in the critically ill trauma and surgical population. JPEN J Parenter Enteral Nutr 2022;46:1191-1197.
- 5. O’Keefe GE, Shelton M, Cuschieri J, Moore EE, Lowry SF, Harbrecht BG, Maier RV. Inflammation and the Host Response to Injury Collaborative Research Program. Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core--standard operating procedures for clinical care VIII--nutritional support of the trauma patient. J Trauma 2008;65:1520-1528.
- 6. Houdijk AP, Rijnsburger ER, Jansen J, Wesdorp RI, Weiss JK, McCamish MA, Teerlink T, Meuwissen SG, Haarman HJ, Thijs LG, van Leeuwen PA. Randomised trial of glutamine-enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet 1998;352:772-776.